You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
Early detection and treatment of oral cancer is essential for increasing survival rates. The 5-year survival rate has not improved significantly in the past 30 years.1 For all stages combined, about 82% of persons with oral cavity and pharynx cancer survive 1 year after diagnosis. The 5-year and 10-year relative survival rates are 59% and 48%, respectively.2 In whites, the survival rate is approximately 55%, while in blacks, it is only 31%. Oral cancer is the sixth most common cancer worldwide.3 In the US, it accounts for an estimated 35,000 cases of cancer and about 7,600 deaths annually.2 Oral cavity cancer constitutes about 17,000 of these cases per year and is more common than cervical or ovarian cancer, Hodgkin’s lymphoma, or leukemia.3
The average age at diagnosis is 63 years.3 Approximately 96% of oral cancers are detected above the age of 40, and more than 50% of all cancers occur in persons older than 65.4 However, recent evidence indicates oral cancers are becoming more prevalent in people younger than 40 years.5,6 The lifetime ratio of males to females receiving an oral cancer diagnosis is 2:1, although advancing age changes that ratio to nearly 1:1. However, oral cancers are twice as common in males compared to females.2 The overall incidence of oral cancers has stabilized, relative to the occurrence of newly diagnosed cancers of all oral sites, with absolute numbers increasing only slightly each year.7
More than 90% of these oral-pharyngeal cancers are squamous cell carcinomas (SCC). The remainder includes salivary gland tumors, lymphoma, and sarcoma.8
Often, these malignancies begin as preneoplastic inflammatory lesions, such as leukoplakia, erythroplasia, and erythroleukoplakia. Leukoplakia is a common oral lesion, appearing as a highly phenotypically variable white patch, and may be associated with tobacco and alcohol use, as well as chronic inflammation. When these and other risk factors are present, the risk of malignant transformation to SCC may approach 17%.9,10 These leukoplakia (or other premalignant lesions) may become cancerous, especially if they demonstrate epithelial dysplasia. If epithelial dysplasia is diagnosed, the rate of cancer transformation may become as high as 42%.11 Alterations in host immunity, inflammation, angiogenesis, and metabolism have been noted as prominent clinical features in oral cancers.10,11
Anatomic Sites
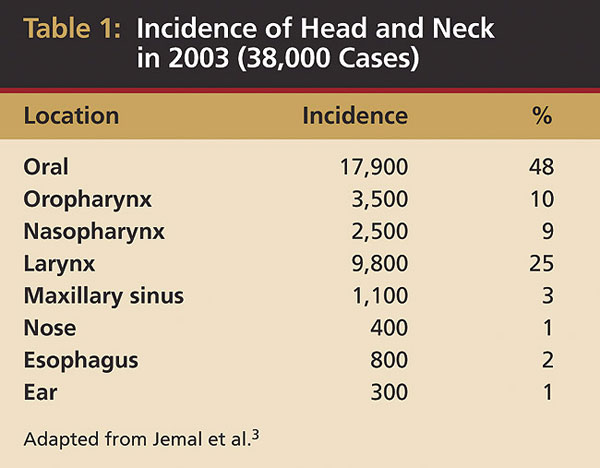
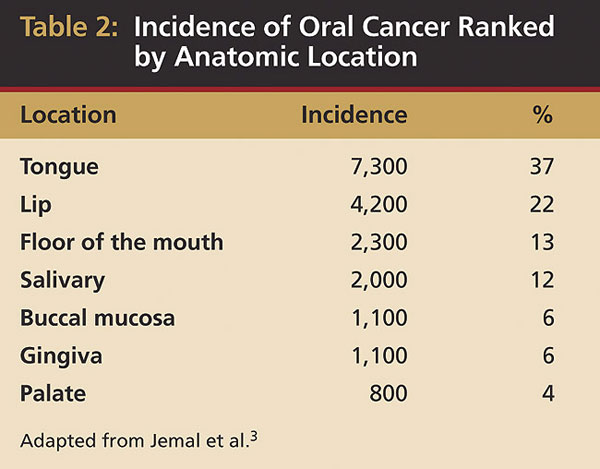
The most common site for oral cancers in both American men and women is the tongue, particularly the posteriorlatero-vental surfaces. Recent data indicate about 37% (7,320:20,010) of all oral cancers, excluding the pharynx, occur on the tongue3 (Table 1). However, populations in other parts of the world experience oral cancers differently: in India, buccal mucosa carcinomas are more common, and in Southeast Asia, nasopharyngeal cancer occurs more frequently. Data from the Surveillance, Epidemiology and End Results (SEER) Program demonstrate that 30% of all oral cancers diagnosed in the US between 1985 and 1996 occurred on the tongue, followed by the lip and floor of the mouth.6,7 Oral tongue malignancies (in the anterior two-thirds) accounted for 53% of tongue cancers.7,8 The other oral anatomic sites are the lip (22%), floor of the mouth (13%), salivary glands (12%), buccal mucosa (6%), gingiva (6%), and palate (4%) (Table 2).3
Stage at Diagnosis and Survival
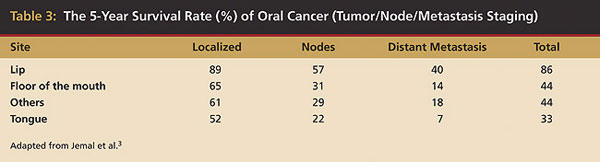
Unfortunately, the overall survival rates for oral and pharyngeal cancers have not improved significantly in the past 30 years. Furthermore, only 60% of these patients will survive 5 years following treatment.1 These statistics are worse for tongue carcinoma: about 33%.7-11 In the US, the outcomes are more favorable for whites than blacks (55% vs 31%, 5-year survival rates). Undoubtedly, genetics are significantly involved in the predisposition to cancer; however, socioeconomic status, education, and access to the healthcare system also have an influence.7-11 The survival rates for advanced tumors are much lower compared with earlier-detected, localized cancers. At diagnosis, almost 50% of all carcinomas of the tongue have metastasized already3 (Table 3). An additional 35% to 40% will do so within 5 years. If all oral cancers were diagnosed and treated early as localized tumors, almost 80% of these patients would have 5-year survival rates.12,13 This is a major reason for the importance of early detection and/or prevention of the premalignant lesion from progressing to carcinoma. Unfortunately, very little progress has been made in the past 40 years regarding early diagnosis. Additionally, based on more than 25,000 SEER Program oral/pharyngeal cases for which adequate information was available, advanced tumors outnumbered localized, early oral cancers by 59% to 41%. The lip was the only major site where localized cancers were found more frequently than more advanced cancers.6-8 Advances in the treatment of oral cancer have not led to significantly improved survival; therefore, earlier diagnosis is the most important factor in improving oral cancer control and reducing morbidity and mortality.7-11
Etiology and Risk Factors
The etiology of oral cancers appears multifactoral, involving long-term exposure to carcinogenic substances, as well as alterations in host immunity and metabolism, angiogenesis, exposure to chronic inflammation, and possibly other factors that accumulate gradually in a genetically susceptible individual. The carcinogenic changes may be influenced by oncogenes, carcinogens, and mutations caused by chemicals, viruses, irradiation, cigarette smoking, excessive alcohol intake, hormones, diet, and physical irritants.9,14,15
Tobacco
Reports from the US Surgeon General and others conclude that cigarette smoking is the main cause of cancer mortality in the US, contributing to an estimated 30% of all cancer-related deaths and substantially to head and neck cancers.6,9,16
The association between cigarette use and oral carcinoma has been firmly established from epidemiologic studies, revealing there are more than twice as many smokers among patients with oral cancers as in control populations.16 One study found that 72% of more than 400 patients with oral cancers smoked, with 58% using more than one pack daily, demonstrating the high risk for tobacco users.17
Tobacco use also increases the already high risk for recurrences of oral cancers as well as second primary oral and pharyngeal cancers.16-18 The combined effects of tobacco and alcohol are illustrated in another study of more than 350 patients who had oral cancers and a mortality rate of 31% within 5 years.19
Alcohol intake also has been associated with the incidence of oral cancers, especially long-term excessive use. One group of investigators found that 44% of 108 patients with cancer of the tongue and 59% of 68 patients with cancer of the floor of the mouth, palate, or tonsillar fossa had unequivocal evidence of alcoholic cirrhosis. Approximately 75% drank alcohol excessively.19 Definitive associations between alcohol-containing mouth rinses and the development of oral cancers have not been established.19
Nutrition
Although some studies indicate a potential association with dietary factors and cancer in general, no clear characteristics, such as deficiencies or excesses of nutrients, have been recognized as directly correlating with cancers of the oral cavity.19,20
Viruses
While the role of viruses in development of oral cancers is not known to cause oral SCC, other head and neck cancers have a defined relationship with viruses. Of the viruses that infect oral tissues, those having oncogenic potential are from two groups: the herpes viruses and papillomaviruses.21-23 The human papilloma viruses, especially type 16, are among the most likely candidates to cause oral cancers—at least in part.21-23 These viruses seem to be more related to pharyngeal cancer than oral cavity sites.
Clinical Examination
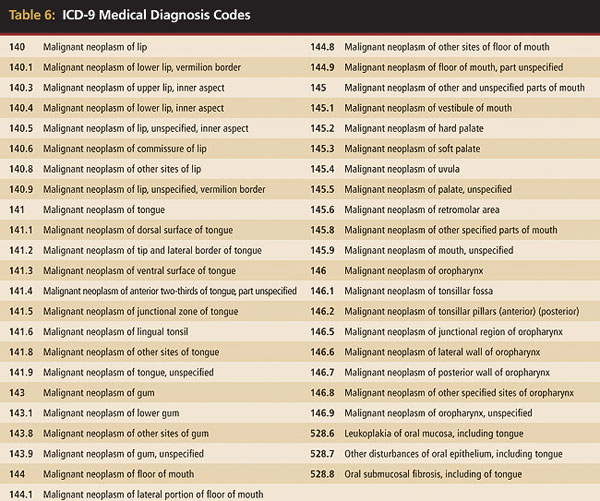
A comprehensive oral examination of every patient is essential to dental practice and for the early detection of oral cancers or premalignant lesions (Table 4 through Table 7). The standard-of-care examination includes not only a thorough inspection of every intraoral mucosal surface but also the extraoral head and neck tissues, including lymph nodes.16,24 Any mucosal abnormality requires an action plan whether that includes treatment, biopsy, referral, or recall examination, and depends upon the nature of the lesion.24 Many oral lesions that are ill-defined, varying in appearance, controversial, and poorly understood may be benign but may present changes that could be confused easily with malignancy. Conversely, early malignancy may be mistaken often for a benign lesion. Some lesions are considered premalignant because of their statistical correlation with subsequent associated cancerous changes.16 It is understandable that a considerable amount of clinical uncertainty is involved in the early detection of malignancy, as well as in the understanding that many of these lesions may not remain benign.


Oral cancers may present clinically with different colors and morphologies. They may appear as leukoplakia (white), erythroplasia (red), and most commonly erythroleukoplakia (red and white). They can also be seen as plaques, macules, ulcers, exophytic papules, nodules or tumors, or granular and/or verrucous lesions. Often, SCCs present with very pleomorphic characteristics—combining several of these features—and may be fissured, indurated, and bleeding.16,24-27
Delay in Diagnosis
In a 2003 retrospective study, Rhodus and Haws found only 14.1% of dentists who had diagnosed epithelial dysplasia (considered premalignant) performed a second biopsy in a 3-year period28 (Table 8).
Often the diagnosis of oral cancer may be delayed because a clinician did not suspect the malignant nature of the lesion and either did not treat or treated inadequently.16,25,26 Most reports reveal that patients usually postpone seeking professional advice for more than 3 months after noticing an oral sign or symptom. Such delays in diagnosis can lead only to local extension of a lesion and increase the risk of metastatic spread of the cancer.16,24,25

Signs and Symptoms
During the earliest stages, oral cancers are usually completely asymptomatic or may present with only mild irritation. Pain usually occurs in the later stages when the lesion advances and ulcerates. Therefore, thorough oral examinations are imperative for detection of the earlier asymptomatic lesions. Although the gold standard for diagnosis is a biopsy and histopathologic examination, visual morphologic changes may aid the decision to biopsy. Ulceration indicates that the lesion has penetrated through the lamina propria into the connective tissue. Rarely, a patient may seek initial consultation because of a swelling in the neck, which represents a metastasis from an oral lesion of which the patient may be completely unaware.16,24,25 Although there are always exceptions, the following are common presenting signs of oral carcinoma:16
- Erythema: Redness of the mucosa that reflects inflammation, thinness, and irregularity of epithelium, as well as a lack of keratinization.
- Ulceration or erosion: Occurs with the destruction of epithelial integrity, owing to discrepancy in cell maturation and disruption of basal lamina (basement membrane).
- Fissuring: The surface texture of the lesion may exhibit ridges and irregularities that reflect abnormal cell growth.
- Induration: Mucosal firmness or hardness because of an increase in the number of epithelial cells secondary to an inflammatory infiltrate.
- Fixation: Abnormally dividing cells invading deeper areas of muscle and bone.
- Chronicity: Failure of lesions to heal. Cancer is not a spontaneously reversible disease. Therefore, a malignant lesion will not disappear normally in the absence of definitive antitumor therapy. It may, however, partially heal or appear to heal, which may fool the clinician.
- Lymphadenopathy: Hardening and/or enlargement of regional nodes because of engorgement with neoplastic cells that spread by lymphatic vessels. Nodes are usually painless and often become fixed because of capsular erosion and local infiltration. Tumors that involve marked induration, fixation, and lymphadenopathy are signs of advanced cancer.
- Leukoplakia: A white patch on the mucosal surface, reflecting excess epithelial keratin production. Hyperkeratosis is associated often with well-differentiated carcinomatous lesions. Excess keratin also may be produced within the stratified squamous epithelium and can appear as “keratin pearls.”
- Erythroplakia: A red macule, plaque, or exophytic lesion that may look similar to trauma or inflammation but may, in fact, represent early angiogenic activity and premaliganancy. The most common clinical presentation of oral precancerous lesions includes some erythema.
- Erythroleukoplakia (mixed): Lesions that present with some combination of both red and white color changes represent the most common clinical appearance of oral precancerous lesions, as well as SCCs.
The risk of subsequent malignant transformation increases when a biopsy specimen reveals an associated epithelial dysplasia. Current scientific consensus is that this transformation occurs in a stepwise fashion through stages of increasingly severe epithelial dysplasia, accompanied by the loss of cell-cycle control, apoptosis, and various genetic aberrations.29-34 However, specific data regarding the correlation among degrees of oral epithelial dysplasia, time-related progression, and the influences of a variety of cofactors remain uncertain.29-33
Oral Carcinogenesis
In oral epithelial tissues, accumulating mutations (eg, genetic progression), chromosomal damage, and loss of cellular control functions are observed during the sequential histologic changes that culminate in oral cancers.29-34 These changes are manifested as the transition from normal histology to early intraepithelial dysplasia and preneoplasia, through increasingly severe intraepithelial neoplasia to superficial cancer, and finally, invasive disease.
When the diagnosis of dysplasia is made, there is no way of determining which dysplasias will transform into carcinoma. At that time, it may be safely assumed severe epithelial dysplasia will most likely proceed to carcinoma in situ, intra-epithelial carcinoma, and/or frank malignancy eventually.
When the dysplasia of the entire epithelium involves disruption of the basal lamina and subsequent invasion of the adjacent connective tissue, the diagnosis of malignancy is certain.
Many studies are being conducted regarding the role of chromosomes and genes in influencing the development and progression of oral leukoplakia to malignancy. Deoxyribonucleic acid (DNA) microarray studies of gene expression are being used to determine the differential profile of genes that are expressed in oral cancers and precancerous (leukoplakia) lesions. Ginos and colleagues at the University of Minnesota found 2,891 genes were expressed differentially in tissues of patients with oral cancers, with categories of genes including those involved in the host immune response, angiogenesis, apoptosis, and cell differentiation among others.35 The same group is using microarrays to analyze not only tissues from premalignant lesions but also in the use of cytologic smears.35 Results from these important studies will aid in identifying risks and prognoses.
Diagnosis and Management
Patients with leukoplakia or other premalignant lesions and even early SCCs are usually asymptomatic.26,36,37 The lesion is usually discovered by a clinician during a routine examination or by a patient who feels roughness in the mouth. No reliable clinical signs and symptoms associated with oral leukoplakia relate to an accurate prediction of a premalignant or early malignant change.9,16,26 However, even mild symptoms are often suggestive of a dysplastic epithelial alteration or an early invasive tumor. Because the clinical appearance of oral leukoplakia—thick or scant, large or small—does not reliably indicate its biologic potential, clinicians should be suspicious of all white lesions and carefully evaluate and observe these patients.9,16,26,36 The diagnosis of these lesions must be made by histopathologic evaluation.
The first step in management of leukoplakia is the removal of all irritants. If the leukoplakia is not reversible, excision is the most effective treatment.26,36,37
Excisional biopsy with subsequent histopathologic examination is the gold standard of diagnosis for oral lesions.26,36,37 However, because these lesions may spread throughout a large area, they cannot always be excised surgically. In many cases, incisional biopsies must be performed and may require multiple-site biopsies in the areas of the lesion that are phenotypically most suspicious for oral cancer.38 In addition, recurrence after excision is common, possibly because of continuation of an irritant or the biologic potential in adjacent tissue with normal morphology (field cancerization). Some areas of the lesion may be cancerous. Therefore, representative sampling is important for the diagnosis. The use of the carbon dioxide laser has proven to be extremely useful and effective.38,39
However, it must be remembered some degree of risk is always present not only for recurrences but also for the development of SCC at the surgical site. Therefore, close follow-up must be emphasized. To help limit recurrences, adjunctive chemotherapy using antiviral medications and antioxidants is being studied.40,41
Although there might be a field keratinization (genomic susceptibility for hyperkeratosis), some evidence of clonal derivation exists. This is seen at times when a portion of a leukoplakic lesion is removed and the residual clinical lesion disappears.29,35
Adjunctive Clinical Diagnostic Aids
While not intended to be diagnostic tests, adjunctive clinical diagnostic aids may benefit clinicians and patients alike when choosing between a scalpel or punch biopsy. These aids may enhance oral mucosal examinations and perhaps facilitate the procurement of a biopsy, which is the gold standard for diagnosing oral pathology.
In a recent comprehensive review in the Journal of the American Dental Association, Patton et al concluded uncertainty persists as to whether adjunctive screening techniques actually improve the numbers of oral cancers diagnosed or the mortality and morbidity associated with them.42 Evidence is insufficient for either supporting or refuting visually based screening adjuncts in dental practice.
However, their review concluded there is data supporting the benefits of toluidine blue vital staining.
Chemiluminescence and Toluidine Blue
Because epithelial dysplasia and early SCC vary considerably in appearance and often resemble certain benign lesions, clinical identification is difficult and biopsy is delayed frequently by attempts at empirical remedies. To better visualize any abnormal oral lesion, clinicians may be able to use the principle of chemiluminescence, which uses a tissue-conditioning technique (with 1% acetic acid) and followed by the use of a fluorescent light (430 nm) (ViziLite® Plus, Zila Pharmaceuticals, Phoenix, AZ), which affects altered tissues and preferentially reflects low energy “blue” light, making the lesion appear very white, or acetowhite. In a study published in 2008 in Oral Oncology, Epstein and colleagues examined 97 clinically suspicious lesions in 84 patients.43 The chemiluminescent examination improved the brightness and/or sharpness of margin in 61.8% of identified lesions. The investigators found chemiluminescence was shown to increase the brightness and margins of mucosal lesions in most cases and therefore may assist in identification of mucosal lesions not considered in traditional visual examination.
However, if the chemilumeniscence is followed with vital staining with tolonium chloride (toluidine blue) applied to the lesion, the resulting stain has been shown to aid early recognition and accelerate biopsy, diagnosis, and treatment.44,45 In the same study by Epstein et al, the toluidine blue stain retention was associated with a large reduction in biopsies showing benign histology (false-positive biopsy results), while maintaining a 100% negative predictive value for the presence of severe dysplasia or cancer.
Toluidine blue is a metachromatic dye of the thiazine group that has been effectively used as a nuclear stain because of its binding to DNA. Overall accuracy of the toluidine blue uptake was 93%.44,45 It can be concluded that toluidine blue staining is a useful adjunct to careful examination, clinical judgment, and biopsy. Abundant evidence indicates that toluidine blue dye used in this diagnostic manner is neither mutagenic nor carcinogenic.44,45
Direct Optical Fluorescence
Instruments based on direct visualization of tissue fluorescence are also used as an aid to identifying diseased tissues. These devices (VELscope®, LED Dental Inc, British Columbia, Canada; Identafi™ 3000, Trimira™, Houston, TX) exploit the properties of naturally occurring fluorophores to characterize biologic tissues. Changes in the tissues’ “optical signature” can be visualized as a loss of normal tissue fluorescence as a lesion progressively becomes dysplastic. The VELscope is being investigated by Dr. Miriam Rosin and colleagues with the BC Cancer Agency in Canada.
Poh et al reported their study of eight patients undergoing surgery after a recent positive biopsy diagnosis of oral SCC.46 Each patient was examined with regular white visible light and direct tissue fluorescence. The clinical lesions were each delineated by direct tissue fluorescence with the VELscope regions (fluorescence visualization loss [FVL] positive) and the surgical margin. Thirty punch biopsies were collected: 18 from FVL-positive areas beyond the clinical margins, three from regions that were FVL but clinically positive, and nine from tissue adjacent to the lesion that was both clinically and FVL negative. FVL was demonstrated in every tumor. In three of the eight tumors, clinical lesions went beyond the FVL area. Biopsies of these clinically positive but fluorescence visualization retained (FVR) sites were all unremarkable histologically (hyperplasia/normal). In contrast, seven of the eight tumors showed FVL areas beyond the clinical lesions.46
A total of 18 biopsies was taken from the clinically normal yet FVL-positive areas and six were cancerous (SCC) (33%); four were severe dysplasia (22%); four (22%) were mild/moderate dysplasia; and four (22%) were hyperplasia/normal.
In the four tumors with FVL-positive areas beyond the general 1-cm surgical margins, three were cancer or severe dysplasia and one was normal/hyperplasia. In biopsies taken from regions judged normal by clinical features and FVR, all nine biopsies were histologically unremarkable (hyperplasia/normal).47
Patton et al remarked, “Both of the studies of the VEL-scope technology were conducted at the same center in patients with known oral dysplasia or SCC confirmed by biopsy and did not involve the use of the [VELscope] technology as an adjunct for the detection or diagnosis of new lesions.”42
This device may be useful in assessing the margins of suspected oral premalignant lesions or oral cancer and therefore may be useful in surgical management. However, no studies have determined these devices as a clinical diagnostic adjunct in lower-risk patients or in a general dental practice.
“Given the variability of fluorescence of the oral mucosa and the high prevalence of benign lesions that may show loss of fluorescence, additional studies are needed.”42
Practitioners may consider using these adjuncts. However, there are few evidence-based studies, all in patients in whom oral lesions had already been identified; these are relatively small studies that include only high-risk patients with suspicious lesions and possibly many false positives that identified other (benign) lesions. No published studies have been performed on regular patients in general practice.
Trasepithelial Cytology and Bush Biopsy
It is not practical or appropriate to biopsy every oral lesion often even after it has been diagnosed as epithelial dysplasia.38,48 A simple, reliable, and acceptable technique to support the health professional’s clinical judgment in differentiating benign lesions from early malignant neoplasia is highly desirable.
This is true especially when the clinician has already obtained a nondiagnostic biopsy and now wishes to follow up the lesion with a noninvasive technique. Exfoliative cytology serves this purpose; however, this technique is an adjunct to, not a substitute for, a scalpel biopsy.16,38 In this technique, individual epithelial cells (ideally including basal cells) are obtained from a lesion, spread on a slide, fixed, stained, and examined by microscopy.38,48 The entire oral cavity is lined with stratified squamous epithelium that varies in thickness and keratinization according to anatomic and functional sites. Transepithelial sampling using a specially designed brush to obtain representative cells from all epithelial layers of a specimen can be accomplished with the proper brush and technique.38,48 The brush biopsy and oral cytology examination can provide a significant adjunct in the evaluation of questionable lesions by giving clinicians an initial screening tool beforehand and indicating the use of scalpel incisional biopsy of lesions that did not appear clinically to be oral cancer. Results from these brush biopsies have indicated the early diagnosis of malignancies that would otherwise have remained unsuspected. A thorough examination of the cell sample is made by an automated microscopic system or by a trained cytologist and certified by a pathologist.38,48
Classification of benign oral diseases from exfoliative cytologic studies is not yet possible; tissue patterns as seen in biopsies are necessary. Patton et al wrote, “There are inconsistencies in specificities and PPVs [positive predictive values] of OralCDx test [OralCDx Laboratories, Inc., Suffern, NY] results across studies.”42 So false-positive and false-negative results are possible, but reports of those have been few. Although undesirable, these errors are manageable because of the manner in which cytologic reports are used and interpreted: 1) if clinical suspicion remains in the face of a negative or atypical report, a biopsy should be performed; 2) a suspicious report indicates a definite need to establish a definitive diagnosis immediately; and 3) when smears contain cells consistent with or suspicious for malignancy, biopsy is mandatory.38,48
Toluidine Blue
Because epithelial dysplasia and early SCC vary considerably in appearance and often resemble certain benign lesions, clinical identification is difficult and biopsy is frequently delayed by attempts at empirical remedies. To better visualize any abnormal oral lesion, clinicians may be able to use a tissue-conditioning technique (with acetic acid) followed by the use of an ultraviolet fluorescent light that makes the lesion appear very white. Following this with vital staining with tolonium chloride (toluidine blue) has been shown to aid early recognition and accelerate biopsy, diagnosis, and treatment.44,45 Toluidine blue is a metachromatic dye of the thiazine group that has been used effectively as a nuclear stain because of its binding to DNA. Overall accuracy of the toluidine blue uptake was 93%.44,45 It can be concluded that toluidine blue staining is a useful adjunct to careful examination, clinical judgment, and biopsy. There is abundant evidence that toluidine blue dye used in this diagnostic manner is neither mutagenic nor carcinogenic.44,45
Chemoprevention
It has been demonstrated that pharmacologic intervention with agents such as retinoids will prevent the progression of precancerous lesions to frank carcinoma in primary leukoplakia and secondary malignancies.49-55 Although several of the various agents studied in this setting were promising in the single-arm setting, only four (all retinoids) have subsequently been confirmed to be effective in randomized studies.49-55 13-cis-retinoic acid has been shown to reverse oral leukoplakia, a common premalignant lesion in the mouth.49-55 The toxicity of particular retinoids used in these trials are of considerable concern, and patients receiving certain agents have a drop-out rate of up to 10% a year, secondary to toxicity.49-55 In addition, patients who have received prior chemotherapy and have hepatic disease are excluded from these trials. A patient with head and neck cancer has a reasonably high likelihood of having one or both of these exclusion criteria. Because the duration of delivery for chemopreventive agents for head and neck cancer remains unclear, the use of compounds with unacceptable long-term adverse toxicity profiles is questionable.49-55
Chemoprevention of oral leukoplakia remains experimental.55,56 However, because of the high potential of effectively treating oral leukoplakia and epithelial dysplasia, multiple clinical trials are underway, including those in the University of Minnesota Preneoplasia Initiative, with several potentially effective chemoprevention agents, such as ketorolac, celecoxib, and pioglitazone.
Follow-Up
Because many methods of managing leukoplakia are not always feasible or effective, these patients must be observed periodically.16,57 The follow-up examinations should be frequent (< 6 months) depending on the actual diagnosis and clinical scenario. In a patient with a persistent lesion, the follow-up interval will be much more frequent (< 4 months). A lesion demonstrating biopsy-proven epithelial dysplasia should have a more frequent follow-up (< 2 months). The follow-ups include careful clinical observation and occasional biopsies,15,57 which are indicated when changes in signs and/or symptoms occur. These changes may be subtle, and adjunctive techniques may be employed to assist in the decision to biopsy.
Exfoliative cytology using the brush biopsy technique and vital staining with toluidine blue help supplement clinical judgment and serve as an adjunct to biopsy. Because the gold standard for diagnosis is tissue biopsy with histopathologic examination, the value of adjunctive techniques is to accelerate microscopic evaluation by indicating the need for biopsy in situations in which a biopsy is delayed or not thought to be indicated or necessary. Negative smears or stains must be balanced with good clinical judgment. Therefore, if clinical suspicion persists regarding a lesion that does not disappear, a standard scalpel biopsy must be considered.16,57
Treatment
Long-term survival and functional results of treatment depend on the tumor stage, histology, and treatment plan.16,57 The treatment plan is developed at pretreatment conferences (tumor boards) by multidisciplinary consultants and subsequent patient and family concurrence. Additional important outcome factors include the patient’s nutritional status, general health, tobacco use, alcohol intake, and likelihood of compliance with the rigors of therapy.58-63
Curative treatment modalities can be local surgery with wide margins, radiation, or a combination of both. Chemotherapy may be used with these modalities to enhance cure rates and preserve function, which has led increasingly to organ preservation strategies. If survival of the patient is in question, the choice may be to just employ palliative measures to ensure pain control and quality of life.58-63
Otolaryngologists, radiation oncologists, dentists, and rehabilitation specialists work cooperatively in the treatment process. The side effects of treatment are permanent and diminish oral function. Treatment planning is based on careful cancer staging and selection of therapies, which allows for prognostication and facilitates the reporting of outcomes. Physical examination, open biopsy, or fine-needle aspiration biopsy, as well as radiologic imaging studies that include computed tomography, magnetic resonance imaging, and positron emission tomography, are used to classify and stage.58-63
Most major functional disabilities following treatment are related to the disease volume, the degree of radiation, and/or chemotherapy required for treatment that relates to the postoperative complications, including the extent of mandible or tissue loss, reduction of tongue mobility, caries and loss of dentition, xerostomia, muscle trismus, diminished taste and mastication, risk of osteoradionecrosis, and anesthesia of the oral cavity. To achieve a cure, the treatment plan considers an adequate resection of the tumor and surrounding normal tissue and the addition of the lymphatic drainage, while attempting to preserve as much normal anatomy and physiology as possible.58-63
References
1. Surveillance Epidemiology and End Results (SEER) Program. SEER Stat Fact Sheets. Oral Cavity and Pharnyx Cancer. National Institutes of Health Web site. http://seer.cancer.gov/statfacts/html/oralcav.html. Accessed March 16, 2009.
2. Cancer Facts and Figures 2008. American Cancer Society Web site. http://www.cancer.org/downloads/STT/2008CAFFfinalsecured.pdf. Accessed March 16, 2009.
3. Jemal A, Tiwari RC, Murray T, et al. Cancer statistics, 2004. CA Cancer J Clin. 2004;54(1):8-25.
4. Edwards BK, Howe HL, Ries LA, et al. Annual report to the nation on the status of cancer, 1973–1999, featuring implications of age and aging on U.S. cancer burden. Cancer. 2002;94(10):2766-2792.
5. Ries LAG, Eisner MP, Kosary CL, et al. SEER Cancer Statistics Review, 1975-2000. Bethesda, MD: National Cancer Institute; 2003.
6. Myers JN, Elkins T, Roberts D, et al. Squamous cell carcinoma of the tongue in young adults: increasing incidence and factors that predict treatment outcomes. Otolaryngol Head Neck Surg. 2000;122(1):44-51.
7. Schantz SP, Yu GP. Head and neck cancer incidence trends in young Americans, 1973-1997, with a special analysis for tongue cancer. Arch Otolaryngol Head Neck Surg. 2002;128(3):268-274.
8. Shiboski CH, Shiboski SC, Silverman S Jr. Trends in oral cancer rates in the United States, 1973-1996. Community Dent Oral Epidemiol. 2000;28(4):249-256.
9. Silverman S Jr. Demographics and occurrence of oral and pharyngeal cancers: the outcomes, the trends, the challenge. J Am Dent Assoc. 2001;132(suppl):7S-11S.
10. Gleich LL, Biddinger PW, Duperier FD, et al. Tumor angiogenesis as a prognostic indicator in T2-T4 oral cavity squamous cell carcinoma: a clinical-pathologic correlation. Head Neck. 1997;19(4):276-280.
11. Pak AS, Wright MA, Matthews JP, et al. Mechanisms of immune suppression in patients with head and neck cancer: presence of CD34+ cells which suppress immune functions within cancers that secrete granulocyte-macrophage colony-stimulating factor. Clin Cancer Res. 1995;1(1):95-103.
12. Landis S, Murray T, Bolden S, et al. Cancer statistics, 1998. CA Cancer J Clin. 1998;48(1):6-29.
13. Vlock DR, Arnold B, Humpierres J, et al. Serial studies of autologous antibody reactivity to squamous cell carcinoma of the head and neck. Cancer Immunol Immunother. 1992;34(5):329-336.
14. Clayman GL, Lippman SM, Laramore GE, et al. Head and neck cancer. In: Holland JF, Frei E, Bast RC Jr, et al, eds. Cancer Medicine. Philadelphia, PA: Williams and Wilkins; 1996:1645-1709.
15. Califano J, van der Riet P, Westra W, et al. Genetic progression model for head and neck cancer: implications for field cancerization. Cancer Res. 1996;56(11):2488-2492.
16. Silverman S Jr. Oral Cancer. Hamilton, London: BC Decker, Inc; 2003:1-128.
17. Forastiere A, Koch W, Trotti A, et al. Head and neck cancer. N Engl J Med. 2001;345(26):1890-1900.
18. Lewin F, Norell SE, Johansson H, et al. Smoking tobacco, oral snuff, and alcohol in the etiology of squamous cell carcinoma of the head and neck: a population-based case-referent study in Sweden. Cancer. 1998;82(7):1367-1375.
19. Mashberg A, Garfinkel L, Harris S. Alcohol as a primary risk factor in oral squamous cell carcinoma. CA Cancer J Clin. 1981;31(3):146-155.
20. Petridou E, Zavras AI, Lefatzis D, et al. The role of diet and specific micronutrients in the etiology of oral carcinoma. Cancer. 2002;94:2981-2988.
21. Miller CS, Johnstone BM. Human papillomavirus as a risk factor for oral squamous cell carcinoma: a meta-analysis, 19821997. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91(6):622-635.
22. Shillitoe EJ, Greenspan D, Greenspan JS, et al. Five-year survival of patients with oral cancer and its association with antibody to herpes simplex virus. Cancer. 1986;58(10):2256-2259.
23. Shillitoe EJ, Gilchrist E, Pellenz C, et al. Effects of herpes simplex virus on human oral cancer cells, and potential use of mutant viruses in therapy of oral cancer. Oral Oncol. 1999;35(3):326-332.
24. Little JW, Falace DA, Miller CS, et al. Dental Management of the Medically Compromised Patient. 6th ed. St. Louis, MO: Mosby, Inc.; 2002:394-412.
25. Axéll T, Pindborg JJ, Smith CJ, et al. Oral white lesions with special reference to precancerous and tobacco-related lesions: conclusions of an international symposium held in Uppsala, Sweden, May 18-21, 1994. International Collaborative Group on Oral White Lesions. J Oral Pathol Med. 1996;25(2):49-54.
26.Lumerman H, Freedman P, Kerpel S. Oral epithelial dysplasia and the development of invasive squamous carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79(3):321-329.
27. Mashberg A, Samit A. Early diagnosis of asymptomatic oral and oropharyngeal squamous cancers. CA Cancer J Clin. 1995;45(6):328-351.
28. Rhodus NL, Haws J, Ondrey F. A follow-up study of 285 oral epithelial dysplastic lesions in Minnesota. Submitted for publication.
29. Forastiere A, Koch W, Trotti A, et al. Head and neck cancer. N Engl J Med. 2001;345(26):1890-1900.
30. Smith BD, Haffty BG, Sasaki CT. Molecular markers in head and neck squamous cell carcinoma: their biological function and prognostic significance. Ann Otol Rhinol Laryngol. 2001;110(3):221-228.
31. Hong WK, Sporn MB. Recent advances in chemoprevention of cancer. Science. 1997;278(5340):1073-1077.
32. Kelloff GJ. Perspectives on cancer chemoprevention research and drug development. Adv Cancer Res. 2000;78:199-334.
33. Kelloff GJ, Sigman CC, Johnson KM, et al. Perspectives on surrogate end points in the development of drugs that reduce the risk of cancer. Cancer Epidemiol Biomarkers Prev. 2000;9(2):127-134.
34. Ondrey FG, Dong G, Van Waes C. Constitutive expression of AP-1 and NF IL-6 in squamous carcinoma of the head and neck. Proc Am Assoc Cancer Res. 1998;39:3081.
35. Ginos MA, Page GP, Michalowicz BS, et al. Identification of a gene expression signature associated with recurrent disease in squamous cell carcinoma of the head and neck. Cancer Res. 2004;64(1):55-63.
36. Silverman S Jr, Gorsky M. Proliferative verrucous leukoplakia: a follow-up study of 54 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;84(2):154-157.
37. Waldron CA, Shafer WG. Leukoplakia revisited. A clinicopathologic study of 3,256 oral leukoplakias. Cancer. 1975;36(4):386-1392.
38. Silverman S Jr. Oral cavity. In: Bibbo M, ed. Comprehensive Cytology. 2nd ed. Philadelphia, PA: WB Saunders; 1997:403-412.
39. Chu FWF, Silverman S Jr, Dedo HH. CO-2 laser treatment of oral leukoplakia: a follow-up of 70 patients. Laryngoscope. 1991;109:950-963.
40. Shaheen NJ, Straus WL, Sandler RS. Chemoprevention of gastrointestinal malignancies with nonsteriodal antiinflammatory drugs. Cancer. 2002;94(4):950-963.
41. Lippman SM, Spitz M, Trizna Z, et al. Epidemiology, biology, and chemoprevention of aerodigestive cancer. Cancer. 1994;74(9 suppl):2719-2725.
42. Patton LL, Epstein JB, Kerr AR. Adjunctive techniques for oral cancer examination and lesion diagnosis: a systematic review of the literature. J Am Dent Assoc. 2008;139(7):896-905.
43. Epstein JB, Silverman S Jr, Epstein JD, et al. Analysis of oral lesion biopsies identified and evaluated by visual examination, chemiluminescence and toluidine blue. Oral Oncol. 2008;44(6):538-544.
44. Portugal LC, Wilson KM, Biddinger PW, et al. The role of toluidine blue in assessing margin status after resection of squamous cell carcinomas of the upper aerodigestive tract. Arch Otolaryngol Head Neck Surg. 1996;122(5):517-519.
45. Warnakulasuriya KA, Johnson NW. Sensitivity and specificity of Orascan® toluidine blue mouthrinse in the detection of oral cancer and precancer. J Oral Pathol Med. 1996;25(3):97-103.
46. Poh CF, Zhang L, Anderson DW, et al. Fluorescence visualization detection of field alterations in tumor margins of oral cancer patients. Clin Cancer Res. 2006;12(22):6716-6722.
47. Poh CF, Ng SP, Williams PM, et al. Direct fluorescence visualization of clinically occult high-risk oral premalignant disease using a simple hand-held device. Head Neck. 2007;29(1):71-76.
48. Christian DC. Computer-assisted analysis of oral brush biopsies at an oral cancer screening program. J Am Dent Assoc. 2002;133(3):357-362.
49. Schaefer SD, Maravilla KR, Suss RA, et al. Magnetic resonance imaging vs computed tomography. Comparison in imaging oral cavity and pharyngeal carcinomas. Arch Otolaryngol. 1985;111(11):730-734.
50. Lippman SM, Benner SE, Hong WK. Cancer chemoprevention. J Clin Oncol. 1994;12(4):851-873.
51. Lippman SM, Lee JS, Lotan R, et al. Biomarkers as intermediate end points in chemoprevention trials. J Natl Cancer Inst. 1990;82(7): 555-560.
52. Hong WK, Endicott J, Itri LM, et al. 13-cis-retinoic acid in the treatment of oral leukoplakia. N Engl J Med. 1986;315(24):1501-1505.
53. Lippman SM, Batsakis JG, Toth BB, et al. Comparison of low-dose isotretinoin with beta carotene to prevent oral carcinogenesis. N Engl J Med. 1993;328(1):15-20.
54. Hong WK, Lippman SM, Itri LM, et al. Prevention of second primary tumors with isotretinoin in squamous-cell carcinoma of the head and neck. N Engl J Med. 1990;323(12):795-801.
55. Benner SE, Pajak TF, Lippman SM, et al. Prevention of second primary tumors with 13cRA in squamous cell carcinoma of the head and neck: long term follow-up. J Nat Cancer Inst. 1994;86:140-141.
56. Sporn MB, Suh N. Chemoprevention of cancer. Carcinogenesis. 2000;21(3):525-530.
57. Mashberg A, Samit A. Early diagnosis of asymptomatic oral and oropharyngeal squamous cancers. CA Cancer J Clin. 1995;45(6):328-351.
58. Fu KK, Pajak TF, Trotti A, et al. A Radiation Therapy Oncology Group (RTOG) phase III randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas: first report of RTOG 9003. Int J Radiat Oncol Biol Phys. 2000;48(1):7-16.
59. Schuchter LM, Hensley ML, Meropol NJ, et al; American Society of Clinical Oncology Chemotherapy and Radiotherapy Expert Panel. 2002 update of recommendations for the use of chemotherapy and radiotherapy protectants: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2002;20(12):2895-2903.
60. Puthawala A, Nisar Syed AM, Gamie S, et al. Interstitial low-dose-rate brachytherapy as a salvage treatment for recurrent head-and-neck cancers: long-term results. Int J Radiat Oncol Biol Phys. 2001;51(2):354-362.
61. Krakoff IH. Systemic treatment of cancer. CA Cancer J Clin. 1996;46(3):134-141.
62. Kramer AM. The role of chemotherapy in head and neck malignancy. Oral Maxillofac Surg Clin North Am. 1993;5:303-517.
63. Merlano M, Vitale V, Rosso R, et al. Treatment of advanced squamous-cell carcinoma of the head and neck with alternating chemotherapy and radiotherapy. N Engl J Med. 1992;327(16):1115-1121.
About the Author
Nelson L. Rhodus, DMD, MPH, Distinguished Professor and Director, Division of Oral Medicine, School of Dentistry, University of Minnesota; Adjunct Professor, Department of Otolaryngology, School of Medicine, University of Minnesota, Minneapolis, Minnesota