You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
Opioid addiction and opioid use disorder are a worldwide crisis, yet the United States consumes the most opioids per capita than any other country in the world.1 In fact, the combined daily opioid dose for every 1 million people in North America (United States and Canada) far eclipses that of any other region, with Germany ranking a distant third place.1 The Centers for Disease Control and Prevention (CDC) determined that overdose deaths from painkillers had more than tripled in the decade leading up to 2016, rising to the level of an epidemic.2 In the year 2000, the CDC estimates there were approximately 20,000 overdose deaths, whereas by 2022 the annual total had skyrocketed to 109,680.3,4
The opioid epidemic in the United States continues to challenge healthcare professionals, prescribers, families, and patients alike.5 Opioid overdose has three hallmark symptoms, frequently referred to as the "opioid overdose triad." These symptoms include shallow, slowed, or stopped breathing; a decreased level of consciousness/responsiveness; and pinpoint pupils.6 Other symptoms of opioid overdose are an undetectable or slowed pulse, very clammy or pale skin, purple or blue fingernails or lips, gurgling noises or vomiting, or a limp body.7 Drug overdoses have become the leading cause of unintentional deaths in the United States, surpassing motor vehicle accidents, with almost 150 lives lost every day.8 Recently published clinical practice guidelines by the CDC to limit the overprescribing, improper use, and availability of prescription opioids should help to stem this tide, but the availability of naloxone to emergency responders, family, friends, and healthcare providers must continue to be a priority.9 A recent publication in the dental literature urged oral healthcare practitioners (OHCPs) to take a leadership role in the face of this epidemic and consider carrying naloxone, an opioid antagonist, in the dental office as the eighth essential medication in the minimal dental emergency kit.10
On March 29, 2023, the US Food and Drug Administration (FDA) deregulated Narcan® (naloxone) 4-mg nasal spray to nonprescription status to increase its availability and access for the general public; it is expected to be sold over-the-counter in supermarkets, vending machines, big-box chains, convenience stores, and gas stations and at online retailers.11,12 Given the anticipated wide availability of naloxone, OHCPs need to be knowledgeable about this potentially lifesaving therapy. The aim of this article is to serve as a primer for dentists regarding the history, pharmacology, appropriate use, and administration of this critically important medication.
Naloxone History and Pharmacology
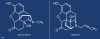
Dr. Jack Fishman originally synthesized naloxone in 1961 while attempting to develop a treatment for constipation subsequent to chronic opioid use.13,14 By replacing the methyl group with an allyl group from the nitrogen atom in oxymorphone, he created a strong competitive and specific antagonist that outcompeted opioids for attachment to receptors, and naloxone was formed (Figure 1).15 In 1971, the FDA approved naloxone under the brand name Narcan© (Adapt Pharma) for use by intramuscular (IM) and intravenous (IV) injection with a recommended initial dose of 0.4 mg,15 and in 1983, the World Health Organization added naloxone to its Model List of Essential Medicines.16,17 Sadly, Dr. Fishman's son, who had struggled with drug use for years, died of an opioid overdose in 2003, almost 10 years before the FDA approved Evzio© (Kaléo Pharma), the first naloxone autoinjector specifically intended for laypeople, and 1 year after Jack's passing.18,19
Naloxone is a neutral ligand and has no clinical effects when given alone, but when administered to patients taking opioid agonists, it competes for the three different opioid receptors in the brain (mu, kappa, and delta), displacing the agonist and reversing its effects (Figure 2).20 Naloxone achieves a brain-to-serum ratio 12 to 15 times greater than morphine.21 In clinical practice it is given to reverse respiratory depression following opioid anesthesia or opioid overdose. In addition, naloxone can reduce or reverse opioid-induced nausea and vomiting, urinary retention, pruritus, and biliary spasm and rigidity associated with numerous opioid therapies.20
The onset of IV naloxone effects occurs in less than 2 minutes, but the half-life and duration of effect are short (30 and 60 minutes, respectively).20 Since respiratory depression from opioids may outlast the effects of naloxone, repeat doses or even a continuous infusion of naloxone may be necessary to maintain reversal of respiratory depression.22-25 Re-narcotization is especially common when using naloxone to reverse longer-acting opioids such as oxycodone and methadone, but may be less of a factor for shorter-acting opioids such as fentanyl.21
In most clinical settings the adult dose of naloxone is 0.4 mg to 2 mg IV, IM, or subcutaneously every 2 to 3 minutes as needed up to a total dose of 10 mg,21 although some literature suggests up to a total dose of 15 mg.22 The FDA-approved dose for children and adolescents is 0.01 mg/kg per dose IV, IM, or subcutaneously every 2 to 3 minutes until the desired response is obtained (Table 1).21 It is unreasonable to expect non-healthcare professionals to be proficient in providing this lifesaving medication at the correct dose either IV, IM, or subcutaneously during an emergency situation, so the FDA approved Evzio for layperson use in 2014; the naloxone autoinjector was available as a twin pack at a fixed dose of 2 mg/0.4 mL per device. Furthermore, the device provided verbal instruction to the user describing how to deliver the medication, similar to automated external defibrillators.
What's Next for Opioid Antagonists?
In 2015, the FDA approved the first intranasal formulation of naloxone (Narcan), designed to deliver a dose of naloxone outside of a healthcare setting in 2-mg and 4-mg fixed doses, thus improving access to emergency treatment and helping to avoid potential needlesticks.26,27 Narcan nasal spray comes as a 2-pack with each individual spray formulation in separate and sealed foil packages that should not be opened until ready for use. In 2019, the first generic intranasal naloxone formulation was approved.28 The intention of these formulations was to make available novel, lifesaving dosages of naloxone that could be easily, safely, and successfully administered by people who are not trained healthcare workers (Table 2).
Using the National Emergency Service Information System, Faul and colleagues found that the percentage of patients requiring multiple sequential naloxone doses increased from 14.5% to 18.2% by emergency medical providers from 2012 to 2015.29 The authors concluded that the increase in multiple doses of naloxone indicated the prevalence of higher-potency opioids. In the New Jersey Emergency Medical system, Klebacher et al examined the incidence of intranasal naloxone redosing from 2014 to 2016 and found that 9% of patients required a second dose and 2% required a third dose.30 Similarly, Marco et al studied emergency department patients with opioid overdoses from 2016 to 2017 and found that the naloxone dose was variable with a median of 4 mg to 8 mg being required.31
Partially based on these trends, in 2021 the FDA approved a high-dose naloxone nasal spray, Kloxxado© (Hikma Pharmaceuticals), which contains 8 mg of naloxone hydrochloride in each 0.1 mL spray.32 More recently, in May 2023, the FDA approved Opvee© nasal spray (nalmefene) (Indivior Inc.) as another emergency treatment of known or suspected opioid overdose in adults and pediatric patients aged 12 years and older.33 While both naloxone and nalmefene act as opioid antagonists, nalmefene has a longer duration of action and may be more useful in managing overdose situations lasting several hours and preventing relapse of overdose symptoms due to re-narcotization. Both Kloxxado and Opvee are prescription-only medications.
All of these formulations help reverse opioid overdose and can save lives. Serious complications are rare, and the benefits of their usage outweigh the risks, although dentists should only consider using Narcan.
What Else Should OHCPs Know About Naloxone?
Naloxone is not classified as a controlled substance, nor is it a substance of abuse. While the injectable formulations are prescription medications in most states, anyone can purchase Narcan nasal spray or the generic 4-mg formulation from a pharmacy without a prescription under collaborative practice agreements or statewide standing orders. California was the first state to deregulate naloxone as an over-the-counter medication in 2014, making it available to the general public for purchase without a prescription.34 Parent groups strongly supported the bill as there was growing concern about the number of young people being lost to unintentional overdoses without access to lifesaving naloxone.34,35 Presently, all 50 states and Washington, DC, allow paramedics to administer naloxone, while many other first responders, such as firemen and police, also carry it.36 These actions have been heralded by the FDA's Center for Drug Evaluation and Research as crucial steps taken to combat the opioid epidemic, in concert with the FDA recently extending the shelf-life of naloxone nasal sprays from 24 to 36 months.10
From a dental regulatory perspective, effective January 2019, Washington State's Dental Quality Assurance Commission was the first to require the co-prescribing of naloxone whenever opioids are prescribed to a high-risk patient for postoperative dental pain.37 The regulation dictates that dentists either refer the patient to a pharmacist for counseling and evaluation or provide a prescription for naloxone.37 Other dental boards are expected to enact similar rules since research continues to suggest that the risk of opioid overdose decreases when clinicians prescribe naloxone along with prescription opioids, even if the patient does not fill the naloxone prescription.8,38 The CDC began recommending naloxone co-prescribing in its 2016 guideline, and in 2018 detailed guidance was issued by the US Department of Health and Human Services.8,39,40 Additional research suggests that naloxone prescription serves as an important educational strategy, and national rates for naloxone-opioid co-prescribing have steadily increased for Medicare Part D insurance plan patients.41 The recently passed Medication Access and Training Expansion (MATE) Act is just one public health policy modification that strives to further reduce inappropriate opioid prescribing and alleviate suffering from opioid overuse.42
Conclusion
The opioid epidemic has a longer history in the United States than most patients and prescribers probably realize. It may take an equally long time to reverse current trends, but strategies to combat this social plague are multifold, and some success is already being recorded. Recently published guidelines to limit the improper use, overprescribing, and availability of prescription opioids have helped to stem this tide, but the availability of naloxone to family, caregivers, emergency responders, and healthcare providers should be a priority. On March 29, 2023, the FDA deregulated some prescription formulations of naloxone nasal sprays to nonprescription status to increase their availability and access for the general public. Given the anticipated wide availability of naloxone, it is important for oral healthcare practitioners to be knowledgeable about this lifesaving therapy.
About the Authors
Mark Donaldson, BSP, ACPR, PharmD
Associate Principal, Vizient Pharmacy Advisory Solutions, Irving, Texas; Clinical Professor, School of Pharmacy, University of Montana, Missoula, Montana; Clinical Assistant Professor, School of Dentistry, Oregon Health & Sciences University, Portland, Oregon; Adjunct Professor, Faculty of Dentistry, University of British Columbia, Vancouver, British Columbia; Fellow, American Society of Health-System Pharmacists; Fellow, American College of Healthcare Executives®
Jason H. Goodchild, DMD
Vice President of Clinical Affairs, Premier Dental Products Co., Plymouth Meeting, Pennsylvania; Associate Clinical Professor, Department of Oral and Maxillofacial Surgery, Creighton University School of Dentistry, Omaha, Nebraska; Adjunct Assistant Professor, Division of Oral Diagnosis, Department of Diagnostic Sciences, Rutgers School of Dental Medicine, New Brunswick, New Jersey
Queries to the author regarding this course may be submitted to authorqueries@broadcastmed.com.
References
1. The International Narcotics Control Board (INCB). Supply of opiate raw materials and demand for opiates for medical and scientific purpose. https://www.incb.org/documents/Narcotic-Drugs/Technical-Publications/2016/NAR_Part_III_Supply_Demand_EN.pdf. Accessed October 31, 2023.
2. Hedegaard H, Warner M, Miniño AM. Drug overdose deaths in the United States, 1999-2016. NCHS Data Brief. 2017;294:1-8.
3. Centers for Disease Control and Prevention. Drug overdose death rates. National Institutes of Health, National Institute on Drug Abuse website. June 30, 2023. https://nida.nih.gov/research-topics/trends-statistics/overdose-death-rates. Accessed October 31, 2023.
4. Centers for Disease Control and Prevention. Provisional data shows U.S. drug overdose deaths top 100,000 in 2022. CDC website. May 18, 2023. https://blogs.cdc.gov/nchs/2023/05/18/7365/. Accessed October 31, 2023.
5. Madras BK. The president's commission on combating drug addiction and the opioid crisis: origins and recommendations. Clin Pharmacol Ther. 2018;103(6):943-945.
6. Schiller EY, Goyal A, Mechanic OJ. Opioid Overdose. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2023.
7. Substance Abuse and Mental Health Services Administration. Opioid overdose. SAMHSA website. Updated October 3, 2023. https://www.samhsa.gov/medications-substance-use-disorders/medications-counseling-related-conditions/opioid-overdose. Accessed November 10, 2023.
8. Centers for Disease Control and Prevention. Opioid overdose. CDC website. August 23, 2023. https://www.cdc.gov/drugoverdose/deaths/opioid-overdose.html. Accessed November 10, 2023.
9. Dowell D, Ragan KR, Jones CM, et al. CDC clinical practice guideline for prescribing opioids for pain - United States, 2022. MMWR Recomm Rep. 2022;71(3):1-95.
10. Goodchild JH, Donaldson M, Malamed SF. Should naloxone be considered an essential medication in dental emergency kits? Gen Dent. 2020;68(3):14-17.
11. US Food and Drug Administration. FDA approves higher dosage of naloxone nasal spray to treat opioid overdose. FDA website. April 30, 2021. https://www.fda.gov/news-events/press-announcements/fda-approves-higher-dosage-naloxone-nasal-spray-treat-opioid-overdose. Accessed October 31, 2023.
12. Hoffman J. F.D.A. approves Narcan for over-the counter sales. March 29, 2023. https://www.nytimes.com/2023/03/29/health/narcan-over-the-counter.html. Accessed October 31, 2023.
13. Campbell ND. Naloxone as a technology of solidarity: history of opioid overdose prevention. CMAJ. 2019;191(34):E945-E946.
14. Andrey-Smith P. Why the inventor of the antidote naloxone lost his stepson to heroin. June 5, 2016. https://www.newsweek.com/jack-fishman-naloxone-opioids-overdose-heroin-466355. Accessed October 31, 2023.
15. RxList.com. Narcan (naloxone hydrochloride injection, USP). Updated October 13, 2022. https://www.rxlist.com/narcan-drug.htm#description. Accessed October 31, 2023.
16. European Monitoring Centre for Drugs and Drug Addiction. Take-home naloxone. EMCDDA website. http://www.emcdda.europa.eu/publications/topic-overviews/take-home-naloxone_en. Accessed October 31, 2023.
17. Lewis CR, Vo HT, Fishman M. Intranasal naloxone and related strategies for opioid overdose intervention by nonmedical personnel: a review. Subst Abuse Rehabil. 2017;8:79-95.
18. US Food and Drug Administration. FDA approves new hand-held auto-injector to reverse opioid overdose. April 3, 2014. http://wayback.archive-it.org/7993/20161022205011/ http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm391465.htm. Accessed October 31, 2023.
19. EVZIO naloxone hydrochloride injection, solution [prescribing information]. Kaléo. https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=5fbe8d17-a72f-406d-a736-48e61620f9d8&type=display. Accessed October 31, 2023.
20. Handal KA, Schauben JL, Salamone FR. Naloxone. Ann Emerg Med. 1983;12(7):438-445.
21. Moss RB, Carlo DJ. Higher doses of naloxone are needed in the synthetic opioid era. Subst Abuse Treat Prev Policy. 2019;14(1):6.
22. Boyer EW. Management of opioid analgesic overdose. N Engl J Med. 2012;367(2):146-155.
23. Wong F, Edwards CJ, Jarrell DH, Patanwala AE. Comparison of lower-dose versus higher-dose intravenous naloxone on time to recurrence of opioid toxicity in the emergency department. Clin Toxicol (Phila). 2019;57(1):19-24.
24. Bailey PL, Clark NJ, Pace NL, et al. Antagonism of postoperative opioid-induced respiratory depression: nalbuphine versus naloxone. Anesth Analg. 1987;66(11):1109-1114.
25. Dahan A, Aarts L, Smith TW. Incidence, reversal, and prevention of opioid-induced respiratory depression. Anesthesiology. 2010;112(1):226-238.
26. Narcan Nasal Spray [prescribing information]. Adapt Pharma. https://s3-us-west-2.amazonaws.com/narcan-assets-uswest/NARCAN-Prescribing-Information.pdf. Accessed October 31, 2023.
27. US Food and Drug Administration. FDA moves quickly to approve easy-to-use nasal spray to treat opioid overdose. November 18, 2015. https://wayback.archive-it.org/7993/20180125101447/ . Accessed October 31, 2023.
28. Pharmacy Times. Barrett J. FDA grants final approval for first generic naloxone nasal spray. April 19, 2019. https://www.pharmacytimes.com/news/fda-grant-final-approval-for-first-generic-naloxone-nasal-spray. Accessed October 31, 2023.
29. Faul M, Lurie P, Kinsman JM, et al. Multiple naloxone administrations among emergency medical service providers is increasing. Prehosp Emerg Care. 2017;21(4):411-419.
30. Klebacher R, Harris MI, Ariyaprakai N, et al. Incidence of naloxone redosing in the age of the new opioid epidemic. Prehosp Emerg Care. 2017;21(6):682-687.
31. Marco CA, Trautman W, Cook A, et al. Naloxone use among emergency department patients with opioid overdose. J Emerg Med. 2018;55(1):64-70.
32. Kloxxado Nasal Spray [package insert]. Hikma Pharmaceuticals. Revised April 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/212045s000lbl.pdf. Accessed October 31, 2023.
33. US Food and Drug Administration. FDA approves prescription nasal spray to reverse opioid overdose. May 22, 2023. https://www.fda.gov/news-events/press-announcements/fda-approves-prescription-nasal-spray-reverse-opioid-overdose. Accessed October 31, 2023.
34. Drug Policy Alliance. Governor Jerry Brown signs overdose law expanding naloxone access in California pharmacies. September 15, 2014. http://www.drugpolicy.org/news/2014/09/governor-jerry-brown-signs-overdose-law-expanding-naloxone-access-california-pharmacies. Accessed October 31, 2023.
35. Collins F. Easier access to naloxone linked to fewer opioid deaths. NIH Director's Blog. May 14, 2019. https://directorsblog.nih.gov/2019/05/14/study-finds-easier-access-to-naloxone-cuts-opioid-deaths. Accessed October 31, 2023.
36. Naloxone intranasal. Medscape website. . Accessed October 31, 2023.
37. Washington State Legislature. Coprescribing of naloxone. WAC 246-817-977. https://app.leg.wa.gov/wac/default.aspx?cite=246-817-977. Accessed October 31, 2023.
38. Tormohlen KN, Schmid I, Stuart EA, et al. State laws that require coprescribing opioids and naloxone and codispensing practices. Am J Prev Med. 2023:S0749-3797(23)00377-X.
39. The Network for Public Health Law. Legal interventions to reduce overdose mortality: naloxone access and overdose Good Samaritan laws. Updated June 2016. https://nosorh.org/wp-content/uploads/2017/05/state-laws-naloxone.pdf. Accessed October 31, 2023.
40. US Dept of Health and Human Services. Naloxone: the opioid reversal drug that saves lives. https://www.hhs.gov/opioids/sites/default/files/2018-12/naloxone-coprescribing-guidance.pdf. Accessed October 31, 2023.
41. Centers for Disease Control and Prevention. National Institute on Drug Abuse. Co-prescribing naloxone in Medicare Part D increases. National Institutes of Health, National Institute on Drug Abuse website. August 6, 2019. https://www.drugabuse.gov/news-events/news-releases/2019/08/co-prescribing-naloxone-in-medicare-part-d-increases. Accessed October 31, 2023.
42. Donaldson M, Goodchild JH. Know your MATE: Medication Access and Training Expansion Act. Gen Dent. 2023;71(5):7-10.