You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
The presence of hard- and soft-tissue defects often poses challenges to implant therapy in the anterior maxilla and can jeopardize the final esthetic outcomes of dental implants.1-3 Vertical ridge augmentation and soft-tissue reconstruction have been recommended to achieve optimal esthetic outcomes with normal crown heights and avoid implant esthetic complications.3-5 Indeed, the lack of augmentation of bone and soft tissue in these case scenarios often results in implants with crowns that either are too long or require pink porcelain, as well as papilla(e) loss and the appearance of black triangles,6-8 which often negatively impacts patients' perceptions of implant therapy.2,6,7,9
The treatment of implant esthetic complications that are characterized by loss of interproximal tissue is also challenging and may require several procedures.7,9-11 Thus, studies have advocated that hard- and soft-tissue augmentation should be performed within the context of implant therapy for reconstruction of an ideal dimension of peri-implant bone, mucosal thickness, and keratinized mucosa to minimize the risk of developing esthetic and biological complications.2-4,12,13
This article describes the rationale and surgical steps for reconstructing the alveolar ridge prior to implant placement in the anterior maxilla and for augmenting the peri-implant soft-tissue phenotype (ie, mucosal thickness, soft-tissue phenotype, and supracrestal tissue height)14 before implant loading.
Case Presentation and Management
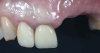
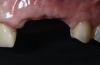
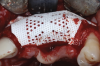
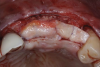
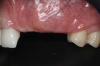
A systemically healthy 40-year-old male patient presented with a maxillary anterior vertical defect (Figure 1 and Figure 2). The patient stated that a previous bone grafting procedure was attempted in this area, but failed. The treatment plan involved a staged guided bone regeneration, the placement of three dental implants 9 months after bone augmentation, and soft-tissue grafting prior to implant loading to augment peri-implant soft-tissue thickness and keratinized mucosa width.
Hard-Tissue Regeneration
A buccal safety flap was performed as has been described previously.13,15 In short, a crestal incision followed by two vertical releasing incisions on the buccal aspects were performed. At the level of the edentulous ridge, the incision was placed not in the middle of the crest, but 2 mm more buccally since the keratinized tissue was abundant. The two slightly divergent incisions were placed two teeth away from the surgical site. It is important to note that a larger flap will provide more access, be easier to close, and result in less mucogingival junction (MGJ) distortion.3-5
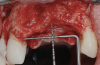
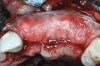
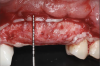
After the primary incisions, periosteal elevators were used to reflect a full-thickness flap beyond the MGJ and at least 5 mm beyond the bone defect (Figure 3). On the palatal aspect, a palatine remote flap, which also has been described previously, was performed.13,15 A sulcular incision followed by two palatal vertical releasing incisions-starting at the distal line angles of the neighboring teeth and extending for approximately 6 mm to 8 mm in length-were executed.
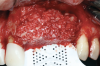
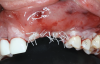
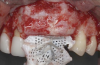
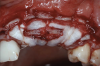
After elevating the buccal and palatal flaps and exposing the bone defect, four cores of autogenous bone were harvested from the retromolar region (Figure 4). These cores were grinded to obtain particulate autogenous bone graft that was mixed with anorganic bovine bone matrix in a 60:40 ratio, respectively. A nonresorbable titanium-reinforced high-density polytetrafluoroethylene (d-PTFE) membrane was trimmed based on the dimension of the defect and fixed to the palatal bone using multiple titanium pins. Then, the mixture of autogenous and xenogeneic bone graft was packed onto the buccal and crestal aspect of the defect, along with the membrane, which was then fixed to the buccal side as well with pins (Figure 5 and Figure 6). At this point, a native collagen membrane was positioned and stabilized over the d-PTFE membrane to form a double layer of membranes over the bone graft (Figure 7). The buccal flap was mobilized with a periosteal releasing incision connecting the two vertical incisions to allow for a tension-free primary closure of the flaps. Multiple horizontal mattress sutures, approximately 4 mm to 5 mm apical to the flap margin, were performed. Simple interrupted sutures were utilized for closing the buccal and palatal flaps at the most coronal aspect and at the level of the vertical incisions (Figure 8).
The postoperative regimen involved the prescription of antibiotics (500 mg of amoxicillin three times a day for 7 days), painkillers (diclofenac, three times a day for 7 days), and 0.12% chlorhexidine mouthrinse daily. The patient was then seen after 3 weeks for suture removal and evaluation of the early surgical outcomes. The healing was uneventful, and the patient was subsequently scheduled for additional follow-up visits.
Implant Placement and Soft-Tissue Augmentation
After 9 months, the site was reopened and the d-PTFE membrane and titanium pins were retrieved (Figure 9). The regenerated bone volume allowed for the placement of three dental implants (parallel, conical connection) in prosthetically driven positions using a surgical guide (Figure 10).
To enhance the esthetic outcome of the implant therapy, a split-thickness flap was opened at the level of the occlusal and buccal aspects of the implants. Two connective tissue grafts (CTGs) were harvested from the palate and sutured over the implants (Figure 11). Two additional CTGs were also obtained and positioned between the implants to augment the peri-implant papillae. This augmentation technique has been described as the "iceberg" connective tissue graft (iCTG) approach (Figure 12).16 The flap was released and sutured to completely cover the CTGs.
This approach was effective in increasing the horizontal and vertical soft-tissue thickness around the three implants. Nevertheless, a distortion of the MGJ was still present (Figure 13). Therefore, 3 months after the iCTG augmentation, it was decided to perform a second soft-tissue augmentation procedure with the goal of repositioning the MGJ in an adequate level on the buccal aspect, increasing the width of the keratinized mucosa at the implant sites. A modified apically positioned flap was performed (Figure 14). Two labial strip gingival grafts were harvested from the anterior mandible and maxilla and sutured onto the recipient bed with a 7-0 polyglycolic acid resorbable suture.5,17,18 The residual periostium was covered with a non-crosslinked xenogeneic collagen matrix (Figure 15).
After 3 months of healing, the second-stage procedure was performed to uncover the dental implants. Then, after 6 months of soft-tissue conditioning with temporary crowns, the case was completed with permanent, implant-supported restorations (Figure 16 and Figure 17).
Discussion
Implant therapy often requires extensive hard- and soft-tissue reconstruction in order to obtain satisfactory outcomes.3,4 The case presented had horizontal and vertical bone deficiency that required staged bone augmentation prior to implant placement and soft-tissue reconstruction to augment the peri-implant soft-tissue phenotype.
One of the main endpoints of implant therapy in the anterior region is patient satisfaction, and this can be highly dependent on the level of the peri-implant soft-tissue margin and the height of the peri-implant papillae.7,8,19 The bone augmentation procedures provided an adequate bone volume and height that enabled the placement of the three dental implants in ideal prosthetically and biologically driven positions. These procedures, however, often result in displacement of the MGJ.5,15,17,18 The first soft-tissue augmentation surgery aimed at increasing soft-tissue thickness in a horizontal and vertical aspect.7,12,20 The iCTG augmentation technique involved augmentation of mucosal thickness on the buccal aspect, which has been demonstrated to have beneficial effects on marginal bone level stability over time,20,21 and augmentation of the interproximal soft tissue, which can result in higher and thicker peri-implant papillae.3,4,10,22 This augmentation procedure requires closure by primary intention to maximize the gain in supracrestal tissue height, and therefore the distortion of the MGJ cannot be corrected with this bilaminar approach. The second soft-tissue augmentation surgery involves an apically positioned flap in combination with two labial strip gingival grafts.5,18 The primary goal of this procedure was in this case to reposition the MGJ on the buccal aspect, increasing keratinized mucosa width at the implant sites.5,18
A cross-sectional study based on surveys demonstrated that dental implants lacking keratinized mucosa were rated by patients to have significantly lower esthetic outcomes compared to implants with an adequate amount of keratinized mucosa width.23 In addition, from a provider perspective, the importance of keratinized mucosa was also evident by the fact that several implant esthetic scores involved the presence/absence of adequate peri-implant keratinized tissue width.19,24,25 Aside from esthetics, keratinized mucosa also has been found to have beneficial effects on peri-implant health-related parameters, such as plaque index, inflammatory scores, and marginal bone levels.20,21,26,27 An adequate band of keratinized and attached ("firm") mucosa improves patients' comfort during brushing and the efficacy of oral hygiene procedures.27,28 It has been advocated that the keratin layer provides mechanical stability and protection to the epithelial cells and underlying tissue.2,27,28
Finally, it should be noted that a recent study from the authors' group found that lack of/inadequate keratinized mucosa was a risk indicator for peri-implant soft-tissue dehiscence in the esthetic zone.29 In other words, having an adequate band of keratinized mucosa promotes better stability of the peri-implant soft tissue, helping to avoid implant esthetic complications.29 The level of the buccal bone compared to the implant platform and mucosal thickness were also found to affect the risk of peri-implant soft-tissue dehiscence, further justifying the need for hard- and soft-tissue augmentation when indicated.29 In particular, it was found that each millimeter increase in buccal bone dehiscence raises the odds of having a peri-implant soft-tissue dehiscence by a factor of approximately 41%.28 Other authors have advocated the importance of buccal bone in the prevention of implant esthetic and biological complications.2,12,30 Therefore, bone augmentation procedures seem to be crucial to not only allow implant placement in the ideal position, but also to prevent future complications.
Lastly, the novel iCTG approach had a positive effect on the peri-implant papillae, where augmentation can otherwise be challenging. The authors propose that a combination of hard- and soft-tissue augmentation, as described in the present report, is a predictable approach for obtaining adequate papillae at implant sites.
Conclusion
Within its limitations, this article described the rationale for and steps involved in hard- and soft-tissue reconstruction of a challenging anterior defect with multiple dental implants. Future prospective cohort studies are needed to further evaluate this approach.
About the Authors
Istvan A. Urban, DMD, MD, PhD
Director, Urban Regeneration Institute, Budapest, Hungary; Adjunct Clinical Associate Professor, Department of Periodontics and Oral Medicine, University of Michigan School of Dentistry, Ann Arbor, Michigan; Visiting Faculty, Harvard School of Dental Medicine, Boston, Massachusetts
Shayan Barootchi, DMD, MS
Adjunct Clinical Assistant Professor, Department of Periodontics and Oral Medicine,University of Michigan School of Dentistry, Ann Arbor, Michigan; Center for
Clinical Research and Evidence Synthesis in Oral Tissue Regeneration (CRITERION),
Boston, Massachusetts
Joseph Y. K. Kan, DDS, MS
Professor, Department of Implantology, Loma Linda University School of Dentistry, Loma Linda, California
Lorenzo Tavelli, DDS, MS, PhD
Assistant Professor, Department of Oral Medicine, Infection, and Immunity, Division of Periodontology, Harvard School of Dental Medicine, Boston, Massachusetts; Center for Clinical Research and Evidence Synthesis in Oral Tissue Regeneration (CRITERION), Boston, Massachusetts
Queries to the author regarding this course may be submitted to authorqueries@broadcastmed.com.
References
1. Hämmerle CHF, Tarnow D. The etiology of hard- and soft-tissue deficiencies at dental implants: a narrative review. J Clin Periodontol. 2018;45 suppl 20:S267-S277.
2. Galarraga-Vinueza ME, Tavelli L. Soft tissue features of peri-implant diseases and related treatment. Clin Implant Dent Relat Res. 2022. doi: 10.1111/cid.13156.
3. Urban IA, Tattan M, Ravida A, et al. Simultaneous alveolar ridge augmentation and periodontal regenerative therapy leveraging recombinant human platelet-derived growth factor-BB (rhPDGF-BB): a case report. Int J Periodontics Restorative Dent. 2022;42(5):577-585.
4. Urban IA, Barootchi S, Tavelli L, Wang HL. Inter-implant papilla reconstruction via a bone and soft tissue augmentation: a case report with a long-term follow-up. Int J Periodontics Restorative Dent. 2021;41(2):169-175.
5. Urban IA, Tavelli L, Barootchi S, et al. Labial strip gingival graft for the reconstruction of severely distorted mucogingival defects: a prospective case series. Int J Periodontics Restorative Dent. 2020;40(6):845-852.
6. Mazzotti C, Stefanini M, Felice P, et al. Soft-tissue dehiscence coverage at peri-implant sites. Periodontol 2000. 2018;77(1):256-272.
7. Tavelli L, Zucchelli G, Stefanini M, et al. Vertical soft tissue augmentation to treat implant esthetic complications: a prospective clinical and volumetric case series. Clin Implant Dent Relat Res. 2023;25(2):204-214.
8. Zucchelli G, Tavelli L, Stefanini M, et al. Classification of facial peri-implant soft tissue dehiscence/deficiencies at single implant sites in the esthetic zone. J Periodontol. 2019;90(10):1116-1124.
9. Tavelli L, Majzoub J, Kauffmann F, et al. Coronally advanced flap versus tunnel technique for the treatment of peri-implant soft tissue dehiscences with the connective tissue graft: a randomized, controlled clinical trial. J Clin Periodontol. 2023. doi: 10.1111/cpe.13806.
10. Stefanini M, Marzadori M, Tavelli L, et al. Peri-implant papillae reconstruction at an esthetically failing implant. Int J Periodontics Restorative Dent. 2020;40(2):213-222.
11. Urban IA, Klokkevold PR, Takei HH. Abutment-supported papilla: a combined surgical and prosthetic approach to papilla reformation. Int J Periodontics Restorative Dent. 2016;36(5):665-671.
12. Wang II, Barootchi S, Tavelli L, Wang HL. The peri-implant phenotype and implant esthetic complications. Contemporary overview. J Esthet Restor Dent. 2021;33(1):212-223.
13. Urban IA, Monje A, Wang HL. Vertical ridge augmentation and soft tissue reconstruction of the anterior atrophic maxillae: a case series. Int J Periodontics Restorative Dent. 2015;35(5):613-623.
14. Avila-Ortiz G, Gonzalez-Martin O, Couso-Queiruga E, Wang HL. The peri-implant phenotype. J Periodontol. 2020;91(3):283-288.
15. Urban IA, Monje A, Nevins M, et al. Surgical management of significant maxillary anterior vertical ridge defects. Int J Periodontics Restorative Dent. 2016;36(3):329-337.
16. Urban I. The Iceberg connective tissue graft. In: Vertical 2: The Next Level of Hard and Soft Tissue Augmentation. Chicago, IL: Quintessence Publishing; 2022.
17. Urban IA, Nagy K, Werner S, Meyer M. Evaluation of the combination of strip gingival grafts and a xenogeneic collagen matrix for the treatment of severe mucogingival defects: a human histologic study. Int J Periodontics Restorative Dent. 2019;39(1):9-14.
18. Urban IA, Lozada JL, Nagy K, Sanz M. Treatment of severe mucogingival defects with a combination of strip gingival grafts and a xenogeneic collagen matrix: a prospective case series study. Int J Periodontics Restorative Dent. 2015;35(3):345-353.
19. Zucchelli G, Barootchi S, Tavelli L, et al. Implant soft tissue dehiscence coverage esthetic Score (IDES): a pilot within- and between-rater analysis of consistency in objective and subjective scores. Clin Oral Implants Res. 2021;32(3):349-358.
20. Tavelli L, Barootchi S, Avila-Ortiz G, et al. Peri-implant soft tissue phenotype modification and its impact on peri-implant health: a systematic review and network meta-analysis. J Periodontol. 2021;92(1):21-44.
21. Thoma DS, Naenni N, Figuero E, et al. Effects of soft tissue augmentation procedures on peri-implant health or disease: a systematic review and meta-analysis. Clin Oral Implants Res. 2018;29 suppl 15:32-49.
22. Rasperini G, Tavelli L, Barootchi S, et al. Interproximal attachment gain: the challenge of periodontal regeneration. J Periodontol. 2021;92(7):931-946.
23. Bonino F, Steffensen B, Natto Z, et al. Prospective study of the impact of peri-implant soft tissue properties on patient-reported and clinically assessed outcomes. J Periodontol. 2018;89(9):1025-1032.
24. Mancini L, Barootchi S, Thoma DS, et al. The peri-implant mucosa color: a systematic appraisal of methods for its assessment and clinical significance. Clin Implant Dent Relat Res. 2023;25(2):224-240.
25. Stefanini M, Felice P, Mazzotti C, et al. Esthetic evaluation and patient-centered outcomes in single-tooth implant rehabilitation in the esthetic area. Periodontol 2000. 2018;77(1):150-164.
26. Lin GH, Chan HL, Wang HL. The significance of keratinized mucosa on implant health: a systematic review. J Periodontol. 2013;84(12):1755-1767.
27. Souza AB, Tormena M, Matarazzo F, Araujo MG. The influence of peri-implant keratinized mucosa on brushing discomfort and peri-implant tissue health. Clin Oral Implants Res. 2016;27(6):650-655.
28. Perussolo J, Souza AB, Matarazzo F, et al. Influence of the keratinized mucosa on the stability of peri-implant tissues and brushing discomfort: a 4-year follow-up study. Clin Oral Implants Res. 2018;29(12):1177-1185.
29. Tavelli L, Barootchi S, Majzoub J, et al. Prevalence and risk indicators of midfacial peri-implant soft tissue dehiscence at single site in the esthetic zone: a cross-sectional clinical and ultrasonographic study. J Periodontol. 2022;93(6):857-866.
30. Monje A, Chappuis V, Monje F, et al. The critical peri-implant buccal bone wall thickness revisited: an experimental study in the beagle dog. Int J Oral Maxillofac Implants. 2019;34(6):1328-1336.