You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
Implant therapy is a predictable and reliable option for replacing missing or hopeless dentition. However, several biologic- and prosthetic-related complications may occur as a result of, or concomitant to, implant therapy.1 Among them is the fact that implant esthetic complications are usually very noticeable to patients.2 These conditions (also known as peri-implant soft-tissue dehiscences [PSTDs]) are characterized by the apical position of the peri-implant soft-tissue margin compared to the cementoenamel junction of the homologous natural tooth.3
PSTDs can impair patients' perception of implant therapy and possibly leave a negative impact on their quality of life.3,4 Several risk factors for the occurrence of PSTDs have been identified and include inadequate amounts of keratinized mucosa and soft-tissue thickness, buccally positioned implants, thin buccal bone, bony dehiscence or fenestrations, and multiple adjacent dental implants.5-7 In particular, a recent study from among the present authors found that each millimeter increase in buccal bone dehiscence raises the odds of having a PSTD by a factor of approximately 41%.7
Several approaches have been described for the treatment of PSTDs, including the use of a coronally advanced flap (CAF) in combination with a connective tissue graft (CTG), tunnel technique approaches (TUN), free gingival grafts, guided bone regeneration procedures, the surgical-prosthetic approach, and vertical soft-tissue augmentation using the CTG-platform approach.4,8-13 The treatment rationale for these approaches largely depends on the patient's expectations and financial capabilities, along with the characteristics of the implant esthetic complication, including but not limited to the height of the implant-supported crown, the buccolingual position of the implant, and the dimension of the papillae. The present authors' group recently introduced a classification for PSTDs as well as recommendations for their treatment.3 Four major classes (I, II, III, and IV) were identified based on the buccolingual position of the implant crown/platform, with three subclasses (a, b, and c), which relate to the height of the peri-implant papillae.3 More complex case scenarios include multiple adjacent implants with PSTDs.
This article describes a case of two dental implants in the anterior maxilla with soft-tissue dehiscences that were treated with CAF + CTG using a submerged approach.
Case Presentation and Treatment Plan
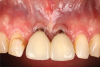
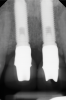
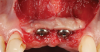
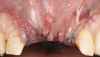
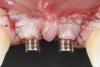
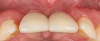
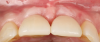
A 46-year-old systemically and periodontally healthy woman presented to the Graduate Periodontics Clinic at the University of Michigan School of Dentistry with the chief complaint of poor esthetics of her smile due to two dental implants (Nos. 8 and 9) showing their metal components (Figure 1). The patient reported that the implants had been placed 15 to 20 years previously after an accident. Clinical examination revealed that the implants had probing depths within 3 mm (facial probing depth of 3-2-3 mm for implant No. 8 and 2-2-2 mm for implant No. 9), no bleeding on probing, and no suppuration; the implants were therefore diagnosed as healthy (Figure 1 and Figure 2). The papilla between the two implants was deficient on the buccal aspect. The implants were buccally positioned, and a substantial lack of soft-tissue volume was noted.
The treatment plan was formulated and discussed with the patient and included removal of the implant-supported crowns and abutments and a surgical procedure involving CAF + CTG with a submerged healing.
Coronally Advanced Flap and Connective Tissue Graft With Submerged Healing
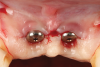
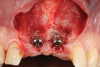
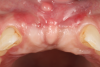
Oral hygiene instructions were reviewed with the patient and reinforced 6 weeks before the surgical visit. At the surgical appointment, the implant-supported crowns and abutments were removed, and two cover screws were inserted. A split-thickness trapezoidal-shape CAF was performed (Figure 3). The two slightly divergent beveled oblique incisions were placed between the implants and lateral incisors. A split-thickness flap was elevated, with meticulous care taken to maintain a layer of connective tissue adherent to the implant surface. Maintaining this layer of connective tissue on the implant body lacking buccal bone is an important step that differentiates flap elevation on healthy implants with PSTDs from implants with peri-implantitis (Figure 4).
After this elevation, the flap was released with a deep incision parallel to the bone and then with a superficial incision with the blade parallel to the external mucosal surface, until it was able to passively reach a position approximately 2 mm coronal to the ideal final level of the soft-tissue margin. The anatomical papillae between the implants and lateral incisors and the inter-incisive papilla were de-epithelialized with a microblade, not only on the buccal aspect but also in the occlusal region to enhance the vascular bed for the flap.
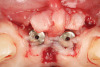
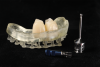
A free gingival graft was harvested from the palate and then extraorally de-epithelialized to obtain a dense and fibrous CTG (Figure 5). A collagen sponge was applied on the palatal donor site and stabilized with 5-0 polytetrafluoroethylene (PTFE) suture. A thin layer of cyanoacrylate tissue glue was applied on top of the collagen sponge, as described by Tavelli et al in 2019.14 No chemical or mechanical root conditioning agents were applied on the implant surface. The CTG was stabilized with simple interrupted sutures (7-0 polyglycolic acid [PGA], butterfly) to the de-epithelialized anatomical papillae on the coronal aspect and the periosteum on its apical portion (Figure 6). The flap was then coronally advanced and sutured by performing simple interrupted sutures (6-0 and 7-0 polypropylene) at the level of the vertical incisions and at the level of the palatal aspect, to completely cover the graft and cover screws (Figure 7).
Sutures were removed after 2 weeks, and the patient reported limited morbidity during the postoperative period.
Reopening of the Site and Restorative Phase
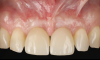
After 3 months of submerged healing, the operated area presented with limited keratinized mucosa on the buccal aspect (Figure 8). Therefore, a flap with two vertical incisions was designed starting from the palatal aspect of the two submerged implants. In addition, the mid-palatal portion of the flap was further extended toward the palatal aspect to obtain a roll flap that was de-epithelialized with a bur and eventually rolled to increase the interproximal soft tissue between the two implants (Figure 9). The flap was elevated in split-thickness fashion, with the soft tissue on top of the implants being removed to identify the cover screws (Figure 10). A substantial increase in soft-tissue thickness was observed at the buccal aspect of the implants compared to the first surgical procedure (Figure 11), when the implant fixtures were visible through a thin layer of connective tissue fibers adherent to the implant surfaces. The cover screws were removed, and temporary titanium abutments were connected.
The flap was closed using simple interrupted sutures (7-0 PGA, butterfly), with the mid-portion of the flap folded and rolled to increase the thickness and height of the interproximal soft tissue between the two implants (Figure 12). 3D-printed temporary crowns were adapted to the flap and delivered (Figure 13). Further modifications of the temporary crowns were performed between 1 month and 3 months, when the final ceramic restorations were delivered.
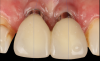
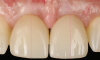
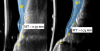
Figure 14 depicts the final outcome at 1 year (which can be compared to the pretreatment photograph in Figure 1). Figure 15 through Figure 18 show clinical comparisons of the outcome at baseline and 1 year, while Figure 19 and Figure 20 provide an ultrasonographic characterization of the soft tissue at baseline and 1 year. The facial probing depths at 1 year were 3-3-3 mm for implant No. 8 and 3-2-3 mm for implant No. 9. The patient was highly satisfied with the esthetic outcomes and overall treatment.
Ultrasonographic Assessment of Mucosal Thickness Gain
Previous studies have described in detail the ultrasound equipment setup and scanning procedures.7,13,15,16 A commercially available ultrasound imaging devicewas used with a 24-MHz miniature-sized transducer to obtain midfacial and interproximal scans at baseline and at 1-year follow-up. The transducer was oriented perpendicular to the occlusal plane and parallel to the implant's long axis to generate "B-mode" grayscale images. B-mode generates 2-dimensional grayscale images in which brightness is the result of the returned echo signal and its strength, which is dependent on the acoustical properties of the periodontal hard- and soft-tissue structures. The scans were saved in digital imaging and communications in medicine (DICOM) files and later exported into a software package, where mucosal thickness was measured 1.5 mm apical to the soft-tissue margin and found to be 0.97 mm and 0.74 mm at the right and left implants, respectively. After 12 months, the respective mucosal thickness was 2.75 mm and 2.48 mm. The soft-tissue thickness gain at the level of the right implant was 1.78 mm for the right implant, and 1.74 mm for the left implant.
Discussion
The efficacy of CAF + CTG for root-coverage procedures in natural dentitions has been well demonstrated.17,18 Nevertheless, when the same "conventional" technique was applied to PSTDs, early studies did not confirm the predictable outcomes observed around natural teeth.4,8 In a pilot case series, Burkhardt and coworkers performed trapezoidal CAF + subepithelial CTG (obtained from the deepest layers of the posterior palate with the single-incision technique) for the treatment of PSTDs.8 Over time, a progressive relapse/shrinkage of the soft tissues with a mean PSTD coverage of 75% at 1 month and 70% at 3 months was observed. The final mean PSTD was 66% at the 6-month follow-up, with none of the sites exhibiting complete PSTD coverage at the last visit.8 The authors suggested that the lack of a periodontal ligament and the overall reduced blood supply that are characteristic of peri-implant tissues compared to natural dentition may have negatively impacted the treatment outcomes.8 Anderson and coworkers randomized 13 patients to either CAF + subepithelial CTG (harvested from the deep palate) or CAF + acellular dermal matrix (ADM). After 6 months, the authors reported a mean PSTD coverage of 40% for CAF + subepithelial CTG and 28% for CAF + ADM. A limited amount of recession coverage was also conveyed by the patients' self-reported esthetics, which did not show a significant improvement 6 months after the treatment.4
Other authors suggested that PSTD treatment should be executed with a CTG harvested either from the most superficial layers of the palate, using a free gingival graft harvesting technique and subsequently removing the epithelium extraorally,19 or from the maxillary tuberosity.20 The palatal soft-tissue harvesting technique used can affect the quality of the graft, with CTGs derived from de-epithelialization of an epithelialized palatal graft being mainly composed of lamina propria, while CTGs from the deep palate obtained with conventional harvesting approaches (eg, deep palate) display higher amounts of glandular and adipose tissue.21 This dissimilar nature of the graft renders a CTG from the superficial palate substantially different than a CTG from the deep palate, with the superficial CTG being firmer, more stable, and easier to manage.21,22 These properties of the CTG may also positively contribute to the keratinization of the overlying epithelium.22 On the other hand, a CTG rich in adipose and glandular tissue, like a deep palate CTG, may act as a barrier and limit the plasmatic diffusion and vascularization during the first phase of healing, and may also impair the ability of the graft to induce keratinization of the overlying epithelium.23 A recent randomized controlled clinical trial from the present authors' group showed that PSTD sites treated with CAF + CTG (obtained from the superficial palate) obtained a mean gain in keratinized mucosa of 2.57 mm at 1 year.13 In addition, CAF + CTG was found to obtain superior clinical, volumetric, and patient-reported outcomes compared to TUN + CTG. After 12 months, the mean PSTD coverage of CAF + CTG and TUN + CTG was 90.23% and 59.76%, respectively.13
Another key consideration is that a substantial difference between treating gingival recessions and PSTDs has to do with the ability to remove the suprastructure at implant sites, when indicated.24 In the described case, the implant-supported crowns and the abutments were removed the day of the surgical procedure. The main advantages of removing the implant suprastructure include improved access for flap design, elevation, and suturing and a larger area that can be de-epithelialized and provide sufficient nutrition and blood supply to the coronally advanced flap.24 The present authors believe that this is a key aspect of treating PSTDs.
In the described clinical case, the implants were slightly buccally positioned, and there was no buccal bone at the midfacial aspect. While the level of the buccal bone (buccal bone dehiscence) has been shown to be a risk indicator for PSTD,7 the treatment of implants with PSTDs lacking buccal bone should be performed with soft-tissue grafting only, and not with bone augmentation.3 A recent study from Quirynen et al showed that the limitations of lateral bone augmentation are dictated by the patient's bony envelope's dimension.25 In other words, when treating implants with PSTDs that are buccally displaced, bone augmentation would have a limited possibility of being successful. Soft-tissue augmentation for the treatment of PSTDs has shown to be an effective approach with stable outcomes over time,3 as described in two 5-year case series.19,20 Indeed, soft-tissue thickness is another important outcome of PSTD treatment that further contributes to the stability of the soft-tissue margin over time.
This article has described the use of real-time, non-ionizing, high-frequency ultrasonography for assessing the increase in soft-tissue thickness and the anatomy of the peri-implant structures pretreatment and at the 1-year follow-up. The reliability and reproducibility of this technology has been shown in several clinical studies in which ultrasonography also was utilized to assess tissue perfusion after graft procedures and the levels of blood flow at healthy versus diseased implant sites.7,13,15,16,26,27 Ultrasonography enabled the observation of a substantial gain of mucosal thickness at 1 year, with the initial soft tissue (measured 1.5 mm apical to the soft-tissue margin) being 0.97 mm and 0.74 mm at the right and left dental implants, respectively, while the final mucosal thickness was 2.75 mm and 2.48 mm at the right and left dental implants, respectively. Considerable evidence supports the key role augmented gingival thickness plays in the stability of the soft-tissue margin in natural dentition,17,28 and it is reasonable to assume that this concept also may be valid at implant sites.13 The benefits of soft-tissue phenotype modification, in terms of increased keratinized mucosa and mucosal thickness, also include patient-reported outcomes and implant health-related parameters.29
Lastly, PSTD treatment is heavily influenced by the patient's desires, expectations, and financial parameters.9,13,24 Therefore, the decision to remove the crown should be discussed with the patient prior to the surgical intervention. Nevertheless, clinicians should be aware that certain types of PSTDs, such as cases characterized by a longer crown with or without pink porcelain at the most apical area or cases with a flat or negative papilla architecture, can only be managed if the patient is willing to replace the crown.3,9,12,24
Conclusion
Peri-implant soft-tissue dehiscences in the esthetic zone are fairly common findings. Correct diagnosis and proper treatment planning are crucial for the management of implant esthetic complications. This article depicted a case of a peri-implant soft-tissue dehiscence treated with a coronally advanced flap and connective tissue graft, with a submerged healing. One year after the treatment, a complete resolution of the defect was observed along with a modification of the peri-implant soft-tissue phenotype and satisfactory esthetic and patient-reported outcomes.
Acknowledgment
The authors thank Gustavo Mendonça, DDS, and Priscila Ceolin Meneghetti, DDS, University of Michigan School of Dentistry, Ann Arbor, Michigan, for their contributions in the restoration of the described case.
About the Authors
Lorenzo Tavelli, DDS, MS, PhD
Assistant Professor, Department of Oral Medicine, Infection, and Immunity, Division of Periodontology, Harvard School of Dental Medicine, Boston, Massachusetts; Center for Clinical Research and Evidence Synthesis in Oral Tissue Regeneration (CRITERION), Boston, Massachusetts
Giovanni Zucchelli, DDS, PhD
Professor of Periodontology and Implantology, Department of Biomedical and Neuromotor Sciences, Bologna University, Bologna, Italy; Adjunct Clinical Professor of Dentistry, Department of Periodontics and Oral Medicine, University of Michigan School of Dentistry, Ann Arbor, Michigan
Hom-Lay Wang, DDS, MSD, PhD
Professor of Periodontics and Dentistry, Department of Periodontics and Oral Medicine, University of Michigan School of Dentistry, Ann Arbor, Michigan
Shayan Barootchi, DMD, MS
Adjunct Clinical Assistant Professor, Department of Periodontics and Oral Medicine, University of Michigan School of Dentistry, Ann Arbor, Michigan; Center for Clinical Research and Evidence Synthesis in Oral Tissue Regeneration (CRITERION), Boston, Massachusetts
Queries to the author regarding this course may be submitted to authorqueries@broadcastmed.com.
References
1. Barootchi S, Ravida A, Tavelli L, Wang HL. Nonsurgical treatment for peri-implant mucositis: a systematic review and meta-analysis. Int J Oral Implantol (Berl). 2020;13(2):123-139.
2. Zucchelli G, Tavelli L, McGuire MK, et al. Autogenous soft tissue grafting for periodontal and peri-implant plastic surgical reconstruction. J Periodontol. 2020;91(1):9-16.
3. Zucchelli G, Tavelli L, Stefanini M, et al. Classification of facial peri-implant soft tissue dehiscence/deficiencies at single implant sites in the esthetic zone. J Periodontol. 2019;90(10):1116-1124.
4. Anderson LE, Inglehart MR, El-Kholy K, et al. Implant associated soft tissue defects in the anterior maxilla: a randomized control trial comparing subepithelial connective tissue graft and acellular dermal matrix allograft. Implant Dent. 2014;23(4):416-425.
5. Romandini M, Pedrinaci I, Lima C, et al. Prevalence and risk/protective indicators of buccal soft tissue dehiscence around dental implants. J Clin Periodontol. 2021;48(3):455-463.
6. Sanz-Martin I, Regidor E, Navarro J, et al. Factors associated with the presence of peri-implant buccal soft tissue dehiscences: a case-control study. J Periodontol. 2020. doi: 10.1002/JPER.19-0490.
7. Tavelli L, Barootchi S, Majzoub J, et al. Prevalence and risk indicators of midfacial peri-implant soft tissue dehiscence at single site in the esthetic zone: a cross-sectional clinical and ultrasonographic study. J Periodontol. 2022;93(6):857-866.
8. Burkhardt R, Joss A, Lang NP. Soft tissue dehiscence coverage around endosseous implants: a prospective cohort study. Clin Oral Implants Res. 2008;19(5):451-457.
9. Mazzotti C, Stefanini M, Felice P, et al. Soft-tissue dehiscence coverage at peri-implant sites. Periodontol 2000. 2018;77(1):256-272.
10. Stefanini M, Marzadori M, Tavelli L, et al. Peri-implant papillae reconstruction at an esthetically failing implant. Int J Periodontics Restorative Dent. 2020;40(2):213-222.
11. Zucchelli G, Mazzotti C, Mounssif I, et al. A novel surgical-prosthetic approach for soft tissue dehiscence coverage around single implant. Clin Oral Implants Res. 2013;24(9):957-962.
12. Tavelli L, Zucchelli G, Stefanini M, et al. Vertical soft tissue augmentation to treat implant esthetic complications: a prospective clinical and volumetric case series. Clin Implant Dent Relat Res. 2023;25(2):204-214.
13. Tavelli L, Majzoub J, Kauffmann F, et al. Coronally advanced flap versus tunnel technique for the treatment of peri-implant soft tissue dehiscences with the connective tissue graft: a randomized, controlled, clinical trial. J Clin Periodontol. 2023. doi: 10.1111/jcpe.13806.
14. Tavelli L, Ravida A, Saleh MHA, et al. Pain perception following epithelialized gingival graft harvesting: a randomized clinical trial. Clin Oral Investig. 2019;23(1):459-468.
15. Barootchi S, Tavelli L, Majzoub J, et al. Ultrasonographic tissue perfusion in peri-implant health and disease. J Dent Res. 2022;101
(3):278-285.
16. Tavelli L, Barootchi S, Rodriguez MV, et al. Recombinant human platelet-derived growth factor improves root coverage of a collagen matrix for multiple adjacent gingival recessions: a triple-blinded, randomized, placebo-controlled trial. J Clin Periodontol. 2022;49(11):1169-1184.
17. Barootchi S, Tavelli L, Zucchelli G, et al. Gingival phenotype modification therapies on natural teeth: a network meta-analysis. J Periodontol. 2020;91(11):1386-1399.
18. Zucchelli G, Mounssif I, Mazzotti C, et al. Coronally advanced flap with and without connective tissue graft for the treatment of multiple gingival recessions: a comparative short- and long-term controlled randomized clinical trial. J Clin Periodontol. 2014;41(4):396-403.
19. Zucchelli G, Felice P, Mazzotti C, et al. 5-year outcomes after coverage of soft tissue dehiscence around single implants: a prospective cohort study. Eur J Oral Implantol. 2018;11(2):215-224.
20. Roccuzzo M, Dalmasso P, Pittoni D, Roccuzzo A. Treatment of buccal soft tissue dehiscence around single implant: 5-year results from a prospective study. Clin Oral Investig. 2019;23(4):1977-1983.
21. Bertl K, Pifl M, Hirtler L, et al. Relative composition of fibrous connective and fatty/glandular tissue in connective tissue grafts depends on the harvesting technique but not the donor site of the hard palate. J Periodontol. 2015;86(12):1331-1339.
22. Tavelli L, Barootchi S, Stefanini M, et al. Wound healing dynamics, morbidity, and complications of palatal soft-tissue harvesting. Periodontol 2000. 2022. doi: 10.1111/prd.12466.
23. Tavelli L, Barootchi S, Greenwell H, Wang HL. Is a soft tissue graft harvested from the maxillary tuberosity the approach of choice in an isolated site? J Periodontol. 2019;90(8):821-825.
24. Zucchelli G, Tavelli L, Stefanini M, et al. The coronally advanced flap technique revisited: treatment of peri-implant soft tissue dehiscences. Int J Oral Implantol (Berl). 2021;14(4):351-365.
25. Quirynen M, Lahoud P, Teughels W, et al. Individual "alveolar phenotype" limits dimensions of lateral bone augmentation. J Clin Periodontol. 2023;50(4):500-510.
26. Barootchi S, Chan HL, Namazi SS, et al. Ultrasonographic characterization of lingual structures pertinent to oral, periodontal, and implant surgery. Clin Oral Implants Res. 2020;31(4):352-359.
27. Tavelli L, Barootchi S, Majzoub J, et al. Ultrasonographic tissue perfusion analysis at implant and palatal donor sites following soft tissue augmentation: a clinical pilot study. J Clin Periodontol. 2021;48(4):602-614.
28. Barootchi S, Tavelli L, Di Gianfilippo R, et al. Soft tissue phenotype modification predicts gingival margin long-term (10-year) stability: longitudinal analysis of six randomized clinical trials. J Clin Periodontol. 2022;49(7):672-683.
29. Tavelli L, Barootchi S, Avila-Ortiz G, et al. Peri-implant soft tissue phenotype modification and its impact on peri-implant health: a systematic review and network meta-analysis. J Periodontol. 2021;92(1):21-44.