You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
Artificial tooth roots (ie, implants) made of titanium have become a standard treatment in modern dentistry for the replacement of missing teeth. With high survival and success rates of up to 98% after 10 years,1 titanium implants represent the gold standard in dental implantology. Since the first dental implants made of titanium were placed in 1965, their number in the United States has risen to 3 million per year,2,3 with approximately 10 million dental implants placed annually worldwide. Soon after titanium implants debuted, Professor Sami Sandhaus introduced the first ceramic implants in 1967.4 For a long time they had an obscure existence and were reserved for holistic dentistry because of the claim of being "metal-free." Due to what was at the time insufficiently stable aluminum oxide material and a lack of surface structuring, these implants were subject to low success rates for many years.5 However, with the introduction of zirconium dioxide in the early 2000s as a reliable dental implant material and the ongoing development of microrough implant surfaces at that time, this began to change.
Today, ceramic implants are increasingly shedding their reputation as a niche product and are progressively finding their way into "conventional" implantology practices. This is partly due to increased health awareness among the population and the resulting rise in demand for metal-free restorations.6 However, the further development of materials, surfaces, and application concepts, and thus increased reliability, is undoubtedly also a major reason for the increased acceptance of ceramic implants on the part of dentists practicing implantology.
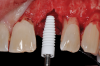
The survival rates of ceramic dental implants have already approached those of titanium implants in the evidence-based indications of "single-tooth restoration" and "three-unit bridge."7 They are on a par with most titanium systems today.8 Although long-term data has yet to confirm these good results, the success so far is encouraging such that clinicians are taking advantage of the clinical benefits of zirconium dioxide in daily practice and using ceramic implants as an additional option in the treatment spectrum alongside titanium implants (Figure 1). Indeed, even titanium, as highly regarded as it is, does not seem to be entirely free of problems.
Comparing Esthetics
The possibility of a gray hue shining through the gingiva is a potential disadvantage of titanium implants. Conversely, because of the white color of zirconium dioxide, this esthetic problem can be avoided with the use of ceramic implants. Of course, achieving excellent esthetics is routinely possible with titanium implants. However, a prerequisite for such an outcome is the presence of a peri-implant mucosa that is at least 2 mm thick, which can prevent the grayish show-through of the titanium implant.9 If this required thickness is not present, the mucosa may be thickened with a connective tissue graft, typically obtained from the palate. However, this requires an additional surgical procedure and, thus, an added burden for the patient. The use of all-ceramic implant abutments is an alternative solution, but micromovements of the hard zirconium dioxide abutment on the softer titanium implant can lead to abrasion and even destruction of the implant-abutment connection.10
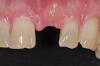
Still, as with titanium implants, undesirable recession with an exposed implant shoulder may occur in rare cases with ceramic implants. Nonetheless, from an esthetic point of view, this inflammation-free recession of the gingiva is likely to be tolerated much better by patients concerned with the dark margins of titanium implants (Figure 2).
The Problem of Peri-implantitis
Even more so than esthetics, the foremost drawback with titanium implants is the risk of peri-implantitis. This inflammatory pathological condition with progressive loss of peri-implant hard and soft tissues can ultimately lead to the loss of the implant. The prevalence of peri-implantitis is significant, with one study determining that almost 15% of implant patients experienced moderate-to-severe peri-implantitis after 9 years.11 Another study found peri-implantitis in 10% of implants and 20% of patients during 5 to 10 years after implantation.12 This prevalence is especially consequential if it is extrapolated to the number of total implants placed. It is, therefore, unsurprising that the etiology of and therapy for peri-implantitis are integral topics at most implantology congresses worldwide and will continue to play increasingly important roles going forward.
Undisputedly, bacterial biofilm (microbial theory) remains a crucial factor in the development of peri-implantitis.13 An accumulation of bacteria and plaque leads to inflammation and tissue recession; good oral hygiene counteracts this. Other risk factors include smoking, diabetes, and local mechanical influences. However, evidence also points to titanium playing a role in this process (foreign body theory).14 Like all metals, titanium is subject to corrosion, and titanium particles have been found in peri-implant bone and soft tissue.15 The distribution of the particles corresponds to the tub-shaped bone defects typical of peri-implantitis.16 This "biocorrosion" is promoted by the use of various metals (eg, gold abutments)17 or the presence of bacterial lipopolysaccharides from bacterial walls.18,19 Intraosseous abrasion from titanium implant particles may also occur as a result of insertion and subsequent functional micromovement of the implant in the bone. These titanium particles are phagocytized in the form of a nonspecific chronic inflammation by local tissue macrophages with secretion of inflammatory cytokines and transported away via the lymphatic system.20 In some patients, the release of pro-inflammatory cytokines appears to be increased compared to a moderate inflammatory response required for regular osseointegration and may be considered locally as a possible additional risk factor of peri-implantitis. This process is a nonspecific, chronic inflammatory reaction that is referred to as titanium intolerance.21
The much cited and often reported "titanium allergy," however, is physiologically not possible. For a metal sensitization (type IV reaction of the late type), the presence of free metal ions is necessary; these bind to the body's own proteins (haptens), change the proteins' structure, and thus can become immunologically reactive in the form of a sensitization. Titanium, however, is highly reactive, and potentially released titanium ions react with oxygen in the millisecond range to form titanium dioxide (passivation layer). Such metal oxides, though, are by definition a "ceramic." Because this reaction is not reversible, no free titanium ions are available for the formation of a possible allergic reaction. A lymphocyte transformation test, which can also be in the form of the still widely used MELISA (memory lymphocyte immunostimulation assay) test, for the detection of titanium sensitization, thus appears to be of little value even though it is still frequently performed.
Most dental implants are made of pure grade IV titanium. However, manufacturing-related impurities associated with nickel or chromium have been detected,22 which, given the known high prevalence of nickel sensitization, is a possible explanation for the so-called "titanium allergies" described in the literature. Other implant systems, like most titanium endoprostheses, are made of a titanium alloy (grade V titanium). Alloy components include aluminum, vanadium, and rodium. In contrast to titanium, these metals may possibly be released in ionic form, thus leading to allergic reactions. Suitable sensitization diagnostics for these components as well as for nickel would thus be indicated.
Ideally, the detection of such an immunological tendency to inflammation should be conducted in advance of implantation, and if this risk constellation is present, a ceramic implant may be considered as a suitable alternative. Because of its material properties, zirconium dioxide is bio-inert, meaning there is no chemical and/or biological interaction between the implant and the tissue, and no toxic or allergenic substances are released. Therefore, sensitization and immunological reactions with regard to zirconium dioxide are eliminated, and intolerance/allergy testing for "ceramics" consequently is not indicated.
Moreover, these bio-inert properties also appear to have benefits with regard to peri-implantitis development and soft-tissue quality. Compared to titanium, zirconium dioxide material shows lower plaque accumulation and bacterial adhesion23,24 as well as a lower thickness of deposited biofilm.25 Circular blood flow to the soft tissues is also more similar to a natural tooth with ceramics versus titanium, which offers significantly reduced blood flow.26 Better blood circulation is known to mean healthier soft tissues, and healthier soft tissues, in turn, result in improved esthetic outcomes. Although long-term evidence is still lacking for ceramic implants, initial 3- and 5-year results are available, and the trend, supported by preclinical studies and clinical experience, is that zirconium dioxide shows the same-and in some studies even less-marginal bone loss as titanium implants. Peri-implantitis has not yet been described clinically in this period.27-30 It should be noted, however, that with ceramic implants, as with titanium implants, cementitis, ie, peri-implant inflammation caused by cement residue, can lead to peri-implant bone defects due to incomplete removal of excess cement.31 Morever, overheating may occur with zirconium dioxide implants when screwing the implant into bone,32 and excessively rough or porous implant surfaces can lead to peri-implant bone defects due to the low thermal conductivity of the material. 33,34
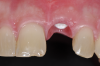
While more scientific evidence showing a lower tendency toward peri-implantitis may still need to be provided for ceramic implants, the essential argument for these implants based on clinical experience is the excellent and almost consistently inflammation-free peri-implant soft-tissue condition (Figure 3).
Zirconia Material and the Composition of Zirconium Dioxide
The bio-inert behavior is due to the material properties of zirconium dioxide as a ceramic. The natural starting material, zircon, is indeed a metal; however, as alluded to earlier, through reaction with oxygen, it is completely oxidized to zirconium dioxide and, thus, irreversibly converted to a ceramic. In contrast to the electrons in the metal titanium, which are still free and therefore reactive, the electrons in the ceramic zirconium dioxide are firmly bound-and, therefore, non-reactive-in genuine covalent bonds. This low reactivity helps produce the special properties of the ceramic: low thermal and electrical conductivity, low surface tension, low solubility/"corrosion," and, thus, less destructive interaction with surrounding tissues. On the other hand, it is also associated with a lack of elasticity and thus higher susceptibility to fracture.
The previous high fracture rates of ceramic implants due to this susceptibility to fracture have been significantly reduced, from 3.4% to 0.2%, as a result of rapid further development of the material properties.35 This improvement has been confirmed in both static and dynamic fracture strength tests according to ISO 14801, which means that some modern ceramic implant systems could also be classified as suitable for clinical use in terms of fracture strength.36,37
Other factors besides the material, however, also play a role in the stability of an implant. For example, in a statement on two-piece ceramic implants, the European Society for Ceramic Implantology said, "The fracture strength and mechanical stability of two-piece zirconia implants may vary depending on different manufacturing processes, material properties, implant geometries, and prosthetic connection concepts."38
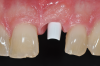
The particular microcrystalline structure of zirconium dioxide is another important factor for stability, and, thus, understanding it is of clinical relevance. The crystals (grains) of zirconium dioxide initially exist in a softer, unstable monoclinic phase (monoclinic crystal lattice). During the manufacturing process, this monoclinic phase is transformed into the desired harder and stable tetragonal phase at a pressure of up to 2000 bar and a temperature of 1170°C (ie, hot isostatic pressing). These crystals at this point are referred to as tetragonal zirconia polycrystals (TZP) (Figure 4). The volume of the crystals is reduced by 3% to 5%. However, this desired tetragonal phase has the tendency to fall back into the monoclonal phase under the influence of energy. This process is called phase transformation and is accompanied by a reduction in density, toughness, and hardness.39
To reduce phase transformation from tetragonal back to monoclonal, stabilizing oxides (yttrium oxide) are added with a volume fraction of 2% to 3%. Yttrium oxide increases the energy required for phase transformation, making phase transformation more difficult. The material at this point is referred to as Y-TZP.40
Phase transformation plays a critical role in everyday clinical practice. This is because, under unfavorable circumstances, grinding of the zirconium dioxide can introduce into the material precisely the energy that initiates the development of a phase transformation; thus, the material is weakened. However, the increase in volume of the monoclonal crystals stops crack propagation (ie, self-healing effect) (Figure 5).41 This may explain why clinicians might not approve of some one-piece implant systems in cases where grinding is involved. In other systems, it has been shown that grinding the implants has no influence on susceptibility to fracture.42
Modern Composite Ceramics and Their Manufacturing Processes
Through additional modification of the composition of ceramic, it was possible to further optimize material properties. Thus, the original TZP has an average flexural strength of 1100 MPa (comparison: titanium 400 MPa, aluminum oxide 550 MPa). The addition of up to 0.5 vol% aluminum oxide further increases this flexural strength to 1200 MPa.43 This is currently referred to as TZP-A, which is used in this form for most of today's ceramic implants.
The latest generation of composite ceramic materials is alumina-toughened zirconia (ATZ). The volume fraction of alumina has been increased to 20%. Alumina serves as a stabilizer and reduces or blocks crack propagation. As a result, the flexural strength has been almost doubled to 2000 MPa and the transformation from the monoclinic to the tetragonal phase has been significantly enhanced.44,45
Alumina is not to be confused with the metallic form of aluminum. It plays no role in the current holistic discussion about metallic aluminum as a potential contributor to neurodegenerative diseases, such as Alzheimer's disease. Here, the same concept applies as previously described: aluminum oxide, as a metal oxide, is by definition a ceramic. Its reaction with oxygen is complete and irreversible; no metallic aluminum can be released. Even if a content of aluminum is indicated in tissue and saliva analyses, it is the immunologically irrelevant oxide of aluminum. In these analyses, it is not the molecular or crystalline incorporation that can be detected, but only the atom itself. Thus, from a general medical point of view, alumina as a ceramic is harmless.
The industrial manufacturing process also has an influence on the long-term success of ceramic implants. Grain size, purity, density, as well as size, quantity, and distribution of possible defects in the material have a significant effect on the hardness and quality of the implant. Two basic processes for manufacturing ceramic implants can be distinguished: production of the implant from a still-soft starting material and from an already-hardened starting material. The first process can be done using one of two methods, either ceramic injection molding (CIM), where shaping is first performed by injection molding,46 or cold isostatic pressing (CIP), which entails milling the still-soft starting material (green compact machining). In both of these methods, the blank is then hardened and refined through sintering, or firing. Both methods are cost-effective, but up to 20% sinter shrinkage must be factored in.47 This shrinkage can lead to higher susceptibility to defects and defect inclusions. In the second manufacturing process, called hard machining, the procedure is reversed: first a block of material is refined (sintered) and only then, at great industrial expense, is the mold ground from the finished, hard blank. This process is more expensive and time-consuming but is capable of yielding very high-quality and precise results (Table 1).
Osseointegration of Ceramic Implants
For long-term success, an implant must be stably osseointegrated, ie, firmly fused with the bone. Osseointegration is defined as a resilient, structural bond between vital bone and the dental implant surface. A measure of the degree of osseointegration is the bone-implant contact (BIC) value. The greater the BIC value, the more stable the implant. Smooth implant surfaces generally have a low BIC, while rough surfaces tend to yield a higher BIC because of an increased surface area and sufficient stimulation for bone formation.48
Thus, the smooth surfaces of early ceramic implants resulted in lower osseointegration and, consequently, higher implant loss rates. Therefore, the goal became the design of rough surfaces to achieve higher BIC, as with titanium implants, and thus improve osseointegration. Ideal surface roughness is 1.0 μm to 2.0 μm,49 and this has already been achieved with ceramic implants.50 However, while the machining of a titanium surface is comparatively simple, when industrial machining a zirconium dioxide surface, potential structural changes in the crystal structure (phase transformation) due to excessive energy input must be avoided. Various surface processing methods, therefore, are used to increase the macro- and microstructure. These methods include, among others, the creation of a rough surface directly over the mold in the CIM process, surface modification by laser, the application of additional coatings, and creation of the macrostructure by sandblasting followed by microstructuring via thermal surface etching with different acids and resulting in different etching patterns (Figure 6). Ultimately, ceramic implants with modern microroughened surfaces have demonstrated in animal studies and various reviews osseointegration rates comparable to titanium implants,51-53 and, thus, similar long-term results can be expected.
Implant Concepts
The dental industry has recognized the development of ceramic implants and their increasing relevance in implantology. Most renowned dental implant suppliers currently include ceramic implants with various restoration concepts in their product portfolios. This has led to greater research expenditure, resulting in increasingly higher-quality products.
One-Piece/Monobloc Implants
Most ceramic implants offered today are one-piece systems, as has been the case since their introduction. Due to their longer history, they have a better evidence and study base than two-piece systems. A one-piece implant effectively solves the dilemma of the implant-abutment connection, as there is no connection; the abutment and implant are made of one piece. The implant is a homogeneous, hermetically sealed block, which makes it possible to handle it as if it were a natural tooth. An advantage of one-piece ceramic implants is that impression-taking and cementation procedures can be similar to working with the crown restoration of a natural tooth. The device, of course, is not a natural tooth but an implant with different aspects than a natural tooth in terms of flexibility, emergence profile, diameter, relation of the crown-root/implant, and anatomy in the gingival/mucosal sulcus.
Restoring one-piece implants can only be achieved by cementing the restoration. This approach lacks the flexibility and reversibility desired in implantology, and it entails the risk of cementitis. That is, if the implant shoulder is placed deeper than 1 mm to 1.5 mm subgingivally, cement residue can no longer be completely and reliably removed.31 Moreover, a precise implant axis (avoidance of phase transformation by grinding), sufficient primary stability, and an unloaded healing phase (for safe osseointegration) must be ensured. Because the implant and abutment are one unit, the wound margins cannot be primarily adapted. Healing takes the form of transmucosal healing. Since primary wound closure is not possible, extended augmentations are only recommended in a two-stage procedure.
Two-Piece Implants
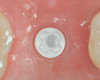
The aforementioned drawbacks of one-piece implants are important reasons why two-piece systems are now considered the standard in modern titanium implantology and why one-piece titanium implants are found only in rare, specific indications. Two-piece systems cover most indications, allow unloaded healing phases as well as safe augmentative procedures after primary wound closure, and are reversible and offer flexibility. As with general implantology, the trend with ceramic implants is, therefore, also toward two-piece systems (Figure 7 through Figure 15), but they still have significantly less evidence and studies compared to one-piece ceramic systems.
The connection of hard, non-elastic zirconium dioxide abutments with hard, non-elastic zirconium dioxide implants still represents a major challenge for two-piece systems. Accordingly, the various manufacturers offer very different connection concepts, which have proven themselves differently with regard to fracture susceptibility and technical complications such as screw loosening.
Bonded Abutment Connection
In two-part cemented ceramic implant systems, the abutment connection thus far has been made by cementing the abutment to the implant. To avoid abutment loosening, only resin-based cements should be used for cementation. The two-piece implant in essence becomes a one-piece implant through abutment bonding after the unloaded healing phase. Consequently, as with a one-piece implant, the restoration also can only be cemented, carries the risk of cementitis, and is no longer reversible or flexible in the event of any needed adjustment.
Screw-Retained Abutment Connection
With ceramic systems, only the screw connection of the abutment and implant is flexible and reversible, as is the case for restoration of titanium implants today. The abutment is fixed in the implant body with the abutment screw. The advantages are obvious: no risk of excess cement, easy soft-tissue management and shaping of the emergence profile, and simple repair and access options.
However, screw retention using earlier commonly used metal screws made of gold or titanium again presents challenges. Ceramics are more resilient in compression than in tension or bending. A screw connection, however, can introduce tensile and bending forces into the implant that are unfavorable for the ceramic material, and this can lead to internal stress peaks. The metal screw is softer than ceramic, and micromovement of the screw in the hard internal implant thread can also cause wear and abrasion to the screw. The precision of the fabrication of the implant-abutment interface and the screw fit are likely to be decisive factors, although further investigation in this area is needed. The tightening torque provided by the manufacturer must be observed in any case. Even if the abutment screw is completely surrounded by ceramic, such solutions are no longer completely metal-free from the point of view of both environmental medicine and the patient. It remains to be confirmed whether abrasion and corrosion by-products can indeed escape via the connecting gap between the abutment and implant.
New approaches are being pursued with metal-free, carbon fiber-reinforced abutment screws that allow a high tightening torque of up to 85 Ncm (clinically applied 25 Ncm). Rounded threads are intended to avoid internal stress peaks.
In general, the two-piece concept offers advantages over its one-piece counterpart in terms of prosthetic flexibility and clinical indications. In both the dental practice and dental laboratory, workflows similar to those used with titanium implants can be retained for handling ceramic implants, which should contribute significantly to further acceptance and spread of them.
Conclusion
Currently available data shows that, due to continued development, ceramic dental implants have approached titanium implants in terms of material stability, surface design, and success rates. To a large extent, familiar surgical and prosthetic protocols can be adopted for ceramic implants. These factors, along with growing evidence and reliability, should lead to greater future acceptance of ceramic implants in dental implantology.
There remain, however, various conceptually different products available on the market, and clinicians have a duty to select the most appropriate implant system for the care of their patients. To achieve long-term clinical success when using ceramic implants and the special features of zirconium dioxide material, it is best to observe the biological principles known from implantology with titanium implants. Manufacturers' guidelines and directives for use in the respective clinical indications must be strictly followed. Assuming these principles and guidance are taken into account, it can be stated that modern ceramic implants, correctly indicated and handled, represent an addition to the treatment spectrum in dental implantology. They will likely continue to become more prevalent in the future.
About the Authors
Jens Tartsch, DMD
President, European Society for Ceramic Implantology; Private Practice,
Zurich, Switzerland
Markus B. Blatz, DMD, PhD
Professor of Restorative Dentistry, Chair, Department of Preventive and Restorative Sciences, and Assistant Dean, Digital Innovation and Professional Development, University of Pennsylvania School of Dental Medicine, Philadelphia Pennsylvania; Editor-in-Chief,Compendium of Continuing Education in Dentistry
Queries to the author regarding this course may be submitted to authorqueries@aegiscomm.com.
References
1. Buser D, Janner SFM, Wittneben JG, et al. 10-year survival and success rates of 511 titanium implants with a sandblasted and acid-etched surface: a retrospective study in 303 partially edentulous patients. Clin Implant Dent Relat Res. 2012;14(6):839-851.
2. Brånemark PI, Adell R, Breine U, et al. Intra-osseous anchorage of dental prostheses. I. Experimental studies. Scand J Plast Reconstr Surg. 1969;3
(2):81-100.
3. Dental Implants Market Size, Share & COVID19 Impact Analysis, UnitedStates, 2021-2027. Burnaby, British Columbia, Canada: iData Research; 2021.
4. Sandhaus S. Technic and instrumentation of the implant C.B.S. (Cristalline Bone Screw) [in Italian]. Inf Odontostomatol. 1968;4(3):19-24.
5. Schulte W, Heimke G. Das Tübinger sofortimplantat [The Tübinger immediate implant] [in German]. Quintessenz. 1976;27(6):17-23.
6. Patient survey. Institut Straumann AG. 2014c. Data on file.
7. Pieralli S, Kohal RJ, Jung RE, et al. Clinical outcomes of zirconia dental implants: a systematic review. J Dent Res. 2017;96(1):38-46.
8. Hashim D, Cionca N, Courvoisier DS, Mombelli A. A systematic review of the clinical survival of zirconia implants. Clin Oral Investig. 2016;20
(7):1403-1417.
9. Jung RE, Sailer I, Hämmerle CHF, et al. In vitro color changes of soft tissues caused by restorative materials. Int J Periodontics Restorative Dent. 2007;27(3):251-257.
10. Stimmelmayr M, Edelhoff D, Güth JF, et al. Wear at the titanium-titanium and the titanium-zirconia implant-abutment interface: a comparative in vitro study. Dent Mater. 2012;28(12):1215-1220.
11. Derks J, Schaller D, Håkansson J, et al. Effectiveness of implant therapy analyzed in a Swedish population: prevalence of peri-implantitis. J Dent Res. 2016;95(1):43-49.
12. Mombelli A, Müller N, Cionca N. The epidemiology of peri-implantitis. Clin Oral Implants Res. 2012;23(suppl 6):67-76.
13. Berglundh T, Armitage G, Araujo MG, et al. Peri-implant diseases and conditions: consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Clin Periodontol. 2018;45(suppl 20):S286-S291.
14. Albrektsson T, Dahlin C, Jemt T, et al. Is marginal bone loss around oral implants the result of a provoked foreign body reaction? Clin Implant Dent Relat Res. 2014;16(2):155-165.
15. Olmedo DG, Tasat DR, Evelson P, et al. Biological response of tissues with macrophagic activity to titanium dioxide. J Biomed Mater Res A. 2008;84(4):1087-1093.
16. He X, Reichl FX, Wang Y, et al. Analysis of titanium and other metals in human jawbones with dental implants - a case series study. Dent Mater. 2016;32(8):1042-1051.
17. Foti B, Tavitian P, Tosello A, et al. Polymetallism and osseointegration in oral implantology: pilot study on primate. J Oral Rehabil. 1999;26(6):495-502.
18. Pettersson M, Kelk P, Belibasakis GN, et al. Titanium ions form particles that activate and execute interleukin-1ß release from lipopolysaccharide-primed macrophages. J Periodontal Res. 2017;52(1):21-32.
19. Yu F, Addison O, Baker SJ, Davenport AJ. Lipopolysaccharide inhibits or accelerates biomedical titanium corrosion depending on environmental acidity. Int J Oral Sci. 2015;7(3):179-186.
20. Jansson V. Distribution of Abrasion Particles of Titanium and Steel in Different Organs. A Comparative In Vivo Study [dissertation]. Munich, Germany: Ludwig Maximilian University; 2007.
21. Jacobi-Gresser E, Huesker K, Schütt S. Genetic and immunological markers predict titanium implant failure: a retrospective study. Int J Oral Maxillofac Surg. 2013;42(4):537-543.
22. Harloff T, Hönle W, Holzwarth U, et al. Titanium allergy or not? "Impurity" of titanium implant materials. Health. 2010;2(4):306-310.
23. Scarano A, Piattelli M, Caputi S, et al. Bacterial adhesion on commercially pure titanium and zirconium oxide disks: an in vivo human study. J Periodontol. 2004;75(2):292-296.
24. Ichikawa Y, Akagawa Y, Nikai H, Tsuru H. Tissue compatibility and stability of a new zirconia ceramic in vivo. J Prosthet Dent. 1992;68(2):322-326.
25. Roehling S, Astasov-Frauenhoffer M, Hauser-Gerspach I, et al. In vitro biofilm formation on titanium and zirconia implant surfaces. J Periodontol. 2017;88(3):298-307.
26. Kajiwara N, Masaki C, Mukaibo T, et al. Soft tissue biological response to zirconia and metal implant abutments compared with natural tooth: microcirculation monitoring as a novel bioindicator. Implant Dent. 2015;24(1):37-41.
27. Cionca N, Hashim D, Cancela J, et al. Pro-inflammatory cytokines at zirconia implants and teeth. A cross-sectional assessment. Clin Oral Investig. 2016;20(8):2285-2291.
28. Balmer M, Spies BC, Vach K, et al. Three-year analysis of zirconia implants used for single-tooth replacement and three-unit fixed dental prostheses: a prospective multicenter study. Clin Oral Implants Res. 2018;29(3):290-299.
29. Janner SFM, Gahlert M, Bosshardt DD, et al. Bone response to functionally loaded, two-piece zirconia implants: a preclinical histometric study. Clin Oral Implants Res. 2018;29(3):277-289.
30. Roehling S, Gahlert M, Janner S, et al. Ligature-induced peri-implant bone loss around loaded zirconia and titanium implants. Int J Oral Maxillofac Implants. 2019;34(2):357-365.
31. Vindasiute E, Puisys A, Maslova N, et al. Clinical factors influencing removal of the cement excess in implant-supported restorations. Clin Implant Dent Relat Res. 2015;17(4):771-778.
32. Zipprich H, Weigl P, König E, et al. Heat generation at the implant-bone interface by insertion of ceramic and titanium implants. J Clin Med. 2019;8(10):1541.
33. Schwarz F, John G, Hegewald A, Becker J. Non-surgical treatment of peri-implant mucositis and peri-implantitis at zirconia implants: a prospective case series. J Clin Periodontol. 2015;42(8):783-788.
34. Kohal RJ, Patzelt SB, Butz F, Sahlin H. One-piece zirconia oral implants: one-year results from a prospective case series. 2. Three-unit fixed dental prosthesis (FDP) reconstruction. J Clin Periodontol. 2013;40(5):553-562.
35. Roehling S, Schlegel KA, Woelfler H, Gahlert M. Performance and outcome of zirconia dental implants in clinical studies: a meta-analysis. Clin Oral Implants Res. 2018;29(suppl 16):135-153.
36. Joda T, Voumard B, Zysset PK, et al. Ultimate force and stiffness of 2-piece zirconium dioxide implants with screw-retained monolithic lithium-disilicate reconstructions. J Prosthodont Res. 2018;62(2):258-263.
37. Spies BC, Fross A, Adolfsson E, et al. Stability and aging resistance of a zirconia oral implant using a carbon fiber-reinforced screw for implant-abutment connection. Dent Mater. 2018;34(10):1585-1595.
38. Scientific Advisory Board and Board of Directors ESCI. Statement of the European Society for Ceramic Implantology (ESCI). The clinical application of two-piece zirconia implants. Zurich, Switzerland: ESCI; June 2021.
39. Chevalier J, Loh J, Gremillard L, et al. Low-temperature degradation in zirconia with a porous surface. Acta Biomater. 2011;7(7):2986-2993.
40. Rieth PH, Reed JS, Naumann AW. Fabrication and flexural strength of ultrafine-grained yttria-stabilized zirconia. Am Ceram Soc Bull. 1976;55(8):717-721.
41. Guazzato M, Albakry M, Ringer SP, Swain MV. Strength, fracture toughness and microstructure of a selection of all-ceramic materials. Part II. Zirconia-based dental ceramics. Dent Mater. 2004;20(5):449-456.
42. Spies BC, Sauter C, Wolkewitz M, Kohal RJ. Alumina reinforced zirconia implants: effects of cyclic loading and abutment modification on fracture resistance. Dent Mater. 2015;31(3):262-272.
43. Metoxit high tech ceramics. Thayngen, Switzerland: Metoxit AG; https://metoxit.com/assets/Downloads/Metoxit-material-overview-en.pdf. Accessed June 27, 2022.
44. Chevalier J. What future for zirconia as a biomaterial? Biomaterials. 2006;27(4):535-543.
45. Schneider J, Begand S, Kriegel R, et al. Low-temperature aging behavior of alumina-toughened zirconia. J Am Ceram Soc. 2008;91(11):3613-3618.
46. Lourenço SH, Coelho R, Vieira MT. Powder injection molding - an excellent micromanufacturing process to produce low-cost zirconia dental implants and abutments. Biomed J Sci Tech Res. 2018;10(3):7771-7772.
47. Yang YS, Chen CY, Chen CS. Effect of injection molding and sintering behaviors on Y-TZP dental implants. J Phys Chem Biophys. 2016;6:3. doi: 10.4172/2161-0398.1000220.
48. Buser D, Schenk RK, Steinemann S, et al. Influence of surface characteristics on bone integration of titanium implants. A histomorphometric study in miniature pigs. J Biomed Mater Res. 1991;25(7):889-902.
49. Wennerberg A, Albrektsson T. Effects of titanium surface topography on bone integration: a systematic review. Clin Oral Implants Res. 2009;20(suppl 4):172-184.
50. Beger B, Goetz H, Morlock M, et al. In vitro surface characteristics and impurity analysis of five different commercially available dental zirconia implants. Int J Implant Dent. 2018;4(1):13.
51. Kohal R. What do we know about zirconia oral implants? A short review including patient case reports. Implantologie. 2014;22(1):9-36.
52. Saulacic N, Erdösi R, Bosshardt DD, et al. Acid and alkaline etching of sandblasted zirconia implants: a histomorphometric study in miniature pigs. Clin Implant Dent Relat Res. 2014;16(3):313-322.
53. Chappuis V, Cavusoglu Y, Gruber R, et al. Osseointegration of zirconia in the presence of multinucleated giant cells. Clin Implant Dent Relat Res. 2016;18(4):686-698.

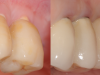



![Fig 7. Restoration and screw-retained abutment with metal-free screw (60% carbon fiber in a polyetheretherketone [PEEK] matrix).](/media/thumbnail/30516)