You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
Alveolar ridge atrophy is a progressive and irreversible phenomenon that is observed following the removal of teeth and which results in a host of esthetic and functional deficiencies.1 While alveolar bone atrophy appears to be directly related to the length of time a tooth has been lost, histologic studies have observed that marked dimensional alterations occur during the first 8 weeks following extraction.2-5 This bone remodeling process is mediated by osteoclasts, which induce resorption of alveolar bone.
Methods to minimize alveolar ridge atrophy, such as bone grafting immediately after tooth extraction, have been extensively used to preserve the alveolar ridge dimensions for future implant placement and subsequent prosthetic rehabilitation. These procedures, however, have focused primarily on maintenance of bone volume with limited attention to bone quality.6-8 Bone quality is negatively affected by residual bone graft material particles that are not replaced by new vital bone.
The aim of this article is to describe the rationale for and technique of implant site preservation with absorbable bone scaffolds to combine preservation of alveolar bone anatomy and quality.
Healing of Extraction Sockets
Alveolar bone resorption has been attributed to disuse atrophy, decreased blood supply, and localized inflammation. An average of 40% to 60% of original height and width of bone is expected to be lost after tooth extraction, with the greatest loss occurring in the first 2 years.9 This can adversely influence bone volume that is needed for future prosthetic rehabilitation. Research has demonstrated that the alveolar ridge at the maxillary anterior area can be reduced by 23% in the first 6 months after tooth extraction, and an additional 11% in the following 5 years.10 In the posterior mandible, resorption happens primarily in the buccal/labial direction, resulting in a lingual displacement of alveolar crest.10 The rate of reduction of residual alveolar ridges has shown to be greater in mandibular arches (0.4 mm per year) than in maxillary arches (0.1 mm per year).11 Thus, alveolar ridge atrophy may prohibit optimal implant placement and compromise the final esthetic and functional outcomes.12
Following tooth extraction, the blood clot that fills the extraction socket is gradually replaced by granulation tissue, which forms the scaffolding for woven bone. Woven bone formation is derived from cell migration from adjacent bony walls. Woven bone is poorly mineralized with a loose trabecular pattern that will later mature into lamellar bone. Traumatic tooth extraction may result in fracture of the surrounding bone, which accelerates bone remodeling.13 Hence, when socket walls are either damaged or missing following tooth extraction, woven bone formation is compromised.13 To minimize alveolar ridge resorption, careful and minimally traumatic tooth extraction should be performed.
Minimally Traumatic Exodontia and Implant Site Preservation
Minimally traumatic tooth extraction begins by disrupting the soft-tissue periodontal attachment apparatus from the tooth. Excessive infiltration of anesthetic agents with vasoconstrictor into the extraction site may negatively affect blood clot formation; thus, bleeding from the site should be encouraged and not avoided.14 Elevation should follow the long axis of the tooth with rotational movements, preventing trauma to adjacent teeth and alveolus. Forceps should be used only after significant mobility of the tooth is achieved. After tooth removal, socket walls must be cleaned with curettes and granulation tissue must be completely removed. Bleeding, if absent, should be stimulated from the osseous base to elicit the formation of a stable blood clot.14
Current methods to prevent ridge resorption include the use of particulate bone grafts and/or barrier membranes.15 Although the use of grafting materials may lead to more positive dimensional outcomes and ridge preservation, the lengthy amount of time necessary for some materials to be replaced by mature, natural bone can be disadvantageous, as residual bone graft material particles may negatively affect bone quality.15 Therefore, the selection of bone replacement materials should be based on the following criteria: (1) be available in unlimited supply; (2) be biologically inert (no immunologic reaction); (3) facilitate revascularization; (4) have osteoconductive capability; and (5) have the potential to be completely replaced by new bone.16-18
The importance of barrier membrane application in combination with bone graft material usage has also been investigated, as this allows for soft-tissue exclusion during the process of bone formation. Barrier membranes are used to not only prevent soft-tissue invasion but also provide a seal over the bone graft material. This seal precludes soft-tissue encapsulation of the most coronal layers of the bone graft material, maximizing the effects of implant site preservation.19-23
Graft Material Selection and Absorbable Bone Scaffold
Anorganic bovine bone has been commonly used during ridge preservation procedures. It is a naturally derived product that maintains a similar crystalline structure, porosity, and carbonate content to human bone mineral, which favors osteoconduction.1 However, it is considered to be a slowly resorbing material due to its high mineral content; therefore, a large number of intact bovine bone particles may remain for years after placement.24 It has been suggested that these residual graft material particles may lead to dental implant complications and treatment failure.25 On the other hand, human-derived bone substitutes have lower mineral content and density compared to bovine bone particles and show only limited residual particles 4 months after placement, which is probably due to their rapid turnover time.26-28
Recent advancements have been made with regard to collagen-based scaffolding with minimal mineral content, to serve as an alternative to traditional bone graft materials. This fully absorbable bone scaffold promotes bone formation without interfering with bone quality by providing a natural environment for cellular migration, proliferation, and maturation. The resultant bone quality is maximized and comparable to natural alveolar bone since no residual material or dense mineralized bone graft material particles remain after a 4-month healing period.29,30 Because this bone replacement material is composed primarily of cross-linked collagen, it can be used to restore intact extraction sockets without a barrier membrane or sutures, but rather is simply sealed with cyanoacrylate adhesive.31
Overview of Technique and Case Presentation
The absorbable bone scaffold technique begins with minimally traumatic exodontia, followed by complete degranulation of the extraction socket. Bleeding from the socket walls is stimulated with the use of curettes. Hydration of the absorbable bone scaffold, consisting of a sterile, biocompatible bone grafting material, is performed. This is followed by adaptation of the absorbable scaffold into the socket without condensation. The absorbable scaffold should be placed to the height of the adjacent soft tissue and sealed with fast-setting cyanoacrylate tissue adhesive. The healing period is a minimum of 4 months before implant placement.
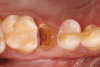
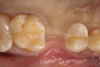
In the case presented, a 56-year-old male patient had a coronal fracture on tooth No. 13, which was deemed hopeless (Figure 1). Treatment options were discussed with the patient, including endodontic therapy followed by surgical crown lengthening with forced tooth eruption to expose sound tooth structure for prosthetic tooth reconstruction. Due to the short length of the residual root, however, an unfavorable prognosis was assigned to this treatment option. The clinician determined, therefore, that replacement of the tooth with a dental implant would be the most conservative, viable, and predictable option for tooth replacement since significant tooth reduction of unrestored adjacent teeth would be necessary for three-unit fixed-bridge fabrication and the patient did not accept any removable replacement options.
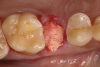
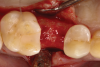
Following local anesthesia via infiltration of 2% xylocaine with 1:100,000 epinephrine, a sulcular incision was carried out on both the buccal and lingual aspects of the tooth to protect adjacent soft tissues during tooth elevation. Periotomes were used following the long axis of the tooth to provide careful elevation of the residual root of the maxillary second premolar. After tooth removal, the extraction socket was inspected and completely degranulated with curettes. A saline solution was used to irrigate the site, and bleeding from the socket walls was stimulated (Figure 2).
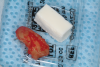
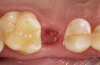
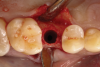
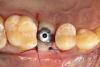
The absorbable bone scaffold material (Figure 3) was quickly hydrated in a sterile saline solution and adapted into the extraction socket with cotton pliers. The material was placed to the level of the adjacent soft-tissue margin, and any excess was removed (Figure 4). Light pressure with a dampened gauze was applied for 1 minute, allowing blood clot formation around the bone scaffold. Fast-setting cyanoacrylate adhesive dressing was used to seal the coronal aspect of the absorbable bone scaffold (Figure 5).
A regularly scheduled postoperative appointment 2 weeks after site preservation revealed adequate wound healing (Figure 6). The patient reported minimal to no discomfort and communicated that the cyanoacrylate layer had fallen out after 10 days, as expected, since the cyanoacrylate material acts as a dressing during early wound healing.
The patient returned 4 months later for implant placement with complete wound healing observed (Figure 7). Following local anesthesia via infiltration of 2% xylocaine with 1:100,000 epinephrine, a crestal incision was carried out over the edentulous area to allow for full-thickness flap elevation (Figure 8). Adequate alveolar bone dimensions for implant placement were observed (Figure 9). Optimal bone density and quality was confirmed during implant osteotomy preparation for placement of a 4.2 mm x 11 mm implant. Optimal insertion torque was recorded (45 Ncm). A healing cap was connected, and flaps were sutured with 4-0 absorbable sutures (Figure 10).
Final intraoral scanning and restorative procedures were completed 3 months later, and the implant has been in function for 36 months at the time of this writing.
Conclusion
Alveolar ridge preservation has proved to be an effective method to prevent or minimize alveolar ridge atrophy following tooth extraction. However, clinicians must also consider the importance of bone quality for long-term stability and success of implant therapy. Hence, it is important to use bone substitutes that not only provide osteoconduction, but also are fully absorbed and replaced with new bone. Controlled clinical trials are needed to further explore absorbable bone scaffolds in not only implant site preservation but also alveolar ridge augmentation procedures.
About the Authors
Rodrigo Neiva, DDS, MS
Chairman and Clinical Professor, Department of Periodontics, University of Pennsylvania School of Dental Medicine, Philadelphia, Pennsylvania
Bruna T. Neiva, DDS, MS
Academic Director, Neiva Institute, Curitiba, Paraná, Brazil
Gisele F. Neiva, DDS, MS
Clinical Professor and Graduate Program Director, Department of Cariology, Restorative Sciences and Endodontics, University of Michigan School of Dentistry, Ann Arbor, Michigan
Queries to the author regarding this course may be submitted to authorqueries@aegiscomm.com.
References
1. Bartee BK. Extraction site reconstruction for alveolar ridge preservation. Part 2: membrane-assisted surgical technique. J Oral Implantol. 2001;27(4):194-197.
2. Araújo MG, Lindhe J. Dimensional ridge alterations following tooth extraction. An experimental study in the dog. J Clin Periodontol. 2005; 32(2):212-218.
3. Cardaropoli G, Araújo M, Hayacibara R, et al. Healing of extraction sockets and surgically produced - augmented and non-augmented - defects in the alveolar ridge. An experimental study in the dog. J Clin Periodontol. 2005;32(5):435-440.
4. Cardaropoli G, Araújo M, Lindhe J. Dynamics of bone tissue formation in tooth extraction sites. An experimental study in dogs. J Clin Periodontol. 2003;30(9):809-818.
5. Araújo MG, Sukekava F, Wennstrom JL, Lindhe J. Ridge alterations following implant placement in fresh extraction sockets: an experimental study in the dog. J Clin Periodontol. 2005;32(6):645-652.
6. Abdulkarim HH, Zeng R, Pazdernik VK, Davis JM. Effect of bone graft on the correlation between clinical bone quality and CBCT-determined bone density: a pilot study. J Contemp Dent Pract. 2021;22(7):756-762.
7. Oliveira MR, Goncalves A, Gabrielli MAC, et al. Evaluation of alveolar bone quality: correlation between histomorphometric analysis and Lekholm and Zarb classification. J Craniofac Surg. 2021;32(6):2114-2118.
8. Yao W, Shah B, Chan HL, et al. Bone quality and quantity alterations after socket augmentation with rhPDGF-BB or BMPs: a systematic review. Int J Oral Maxillofac Implants. 2018;33(6):1255-1265.
9. Polizzi G, Grunder U, Goene R, et al. Immediate and delayed implant placement into extraction sockets: a 5-year report. Clin Implant Dent Relat Res. 2000;2(2):93-99.
10. Artzi Z, Tal H, Dayan D. Porous bovine bone mineral in healing of human extraction sockets: 2. Histochemical observations at 9 months. J Periodontol. 2001;72(2):152-159.
11. Nemcovsky CE, Serfaty V. Alveolar ridge preservation following extraction of maxillary anterior teeth. Report on 23 consecutive cases. J Periodontol. 1996;67(4):390-395.
12. Howell TH, Fiorellini J, Jones A, et al. A feasibility study evaluating rhBMP-2/absorbable collagen sponge device for local alveolar ridge preservation or augmentation. Int J Periodontics Restorative Dent. 1997; 17(2):124-139.
13. Garetto LP, Chen J, Parr JA, Roberts WE. Remodeling dynamics of bone supporting rigidly fixed titanium implants: a histomorphometric comparison in four species including humans. Implant Dent. 1995;4(4):235-243.
14. Wang HL, Tsao YP. Mineralized bone allograft-plug socket augmentation: rationale and technique. Implant Dent. 2007;16(1):33-41.
15. Sclar AG. Strategies for management of single-tooth extraction sites in aesthetic implant therapy. J Oral Maxillofac Surg. 2004;62(9 suppl 2):90-105.
16. Yamada M, Egusa H. Current bone substitutes for implant dentistry. J Prosthodont Res. 2018;62(2):152-161.
17. Simion M, Rocchietta I, Fontana F, Dellavia C. Evaluation of a resorbable collagen matrix infused with rhPDGF-BB in peri-implant soft tissue augmentation: a preliminary report with 3.5 years of observation. Int J Periodontics Restorative Dent. 2012;32(3):273-282.
18. Groger A, Klaring S, Merten HA, et al. Tissue engineering of bone for mandibular augmentation in immunocompetent minipigs: preliminary study. Scand J Plast Reconstr Surg Hand Surg. 2003;37(3):129-133.
19. Duan DH, Wang EB, Zhang Y, Wang HL. Alveolar ridge preservation in severely damaged molar socket using a polylactic acid membrane without primary wound closure: a case series. Int J Oral Maxillofac Implants. 2021;36(6):1224-1234.
20. Zafiropoulos GG, Kacarevic ZP, Qasim SSB, Trajkovski B. Open-healing socket preservation with a novel dense polytetrafluoroethylene (dPTFE) membrane: a retrospective clinical study. Medicina (Kaunas). 2020;56(5):216.
21. Lyu C, Shao Z, Zou D, Lu J. Ridge alterations following socket preservation using a collagen membrane in dogs. Biomed Res Int. 2020;2020:1487681.
22. Cullum D, Lucas M. Minimally invasive extraction site management with dehydrated amnion/chorion membrane (dHACM): open-socket grafting. Compend Contin Educ Dent. 2019;40(3):178-183.
23. Sherif M, Oberic A, Tiple S, Hamedani M. Use of amniotic membrane for covering large oral mucosal defects during socket reconstruction procedures. Klin Monbl Augenheilkd. 2018;235(4):448-449.
24. Valentini P, Bosshardt DD. 20-year follow-up in maxillary sinus floor elevation using bovine-derived bone mineral: a case report with histologic and histomorphometric evaluation. Int J Oral Maxillofac Implants. 2018;33(6):1345-1350.
25. Rodriguez AE, Nowzari H. The long-term risks and complications of bovine-derived xenografts: a case series. J Indian Soc Periodontol. 2019;23(5):487-492.
26. Becker W, Clokie C, Sennerby L, et al. Histologic findings after implantation and evaluation of different grafting materials and titanium micro screws into extraction sockets: case reports. J Periodontol. 1998;69(4):414-421.
27. Demetter RS, Calahan BG, Mealey BL. Histologic evaluation of wound healing after ridge preservation with cortical, cancellous, and combined cortico-cancellous freeze-dried bone allograft: a randomized controlled clinical trial. J Periodontol. 2017;88(9):860-868.
28. Iorio-Siciliano V, Blasi A, Nicolo M, et al. Clinical outcomes of socket preservation using bovine-derived xenograft collagen and collagen membrane post-tooth extraction: a 6-month randomized controlled clinical trial. Int J Periodontics Restorative Dent. 2017;37(5):e290-e296.
29. Zubery Y, Nir E, Goldlust A. Ossification of a collagen membrane cross-linked by sugar: a human case series. J Periodontol. 2008;79(6):1101-1107.
30. Zubery Y, Goldlust A, Alves A, Nir E. Ossification of a novel cross-linked porcine collagen barrier in guided bone regeneration in dogs. J Periodontol 2007;78:112-121.
31. Nevins M, Mendoza-Azpur G, De Angelis N, Kim DM. The biocompatibility of cyanoacrylate tissue adhesive in conjunction with a collagen membrane for providing soft and hard tissue regeneration in extraction socket preservation procedures. Int J Periodontics Restorative Dent. 2018;38(suppl):s37-s42.