You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
Historically, the implant clinician's primary attention was on achieving successful osseointegration. Although Brånemark described this phenomenon as direct apposition of living bone onto the implant (titanium) surface as viewed from the light-microscopic level,1 such observations cannot be made by clinicians, who must therefore rely on alternative, biomechanically oriented definitions of osseointegration. Clinically successful osseointegration has been proposed as, "A process whereby clinically asymptomatic rigid fixation of alloplastic materials is achieved, and maintained, in bone during functional loading."2 Another definition of osseointegration claims a "functional ankylosis,"3 and it is further defined as a direct, structural, and functional connection between ordered, living bone and the surface of a load-bearing implant.4
The alveolar bone has been said to be a product of the tooth and will "remain with the tooth in health."5 More specifically, it can be inferred that, rather than being a product of the tooth, the alveolar bone is an integral structure that, along with the periodontal tissues-specifically the cementum, periodontal ligament (PDL), and bundle bone-helps support the tooth. These seemingly individual components-the alveolar bone, tooth, cementum, and PDL-can be grouped as a singular comprehensive functional unit. Clinicians now understand that unavoidable sequelae occur when a tooth is lost, and when this comprehensive functional unit has one or more critical components removed, resorption of the alveolar ridge will ultimately ensue.6 Therefore, it can be stated that tooth loss and the resulting trauma to the hard tissue is accompanied by multiple dimensional changes and remodeling of the alveolar ridge.7-9
Studies have demonstrated that there is a net resorptive loss of 50% of the alveolar width that occurs in the first 12 months after the extraction of a tooth and associated periodontal tissues.10-12 This is represented by a net deficit of an average of 5 mm to 7 mm of alveolar bone.8 Specifically, the alveolar ridge buccal dimension may experience horizontal resorption of about 56% and a reduction of the lingual/palatal bone wall of 30% during the 4-month period following tooth extraction.9 This is a result of the buccal aspect of the alveolar bone being entirely composed of bundle bone, which itself may be better understood as being calcified PDL fibers.10 Other studies have proposed that two-thirds of this resorption, primarily of the buccal plate, occurs in the first 3 months due to it being less robust than palatal or lingual cortical plates.8 Thus, the resulting morphology of the alveolar ridge post-tooth loss (whether due to trauma, disease, or planned extraction) can present a significant discrepancy in bone height between the lingual and buccal plate,6,13 a decrease in ridge thickness as measured from buccal to palatal or lingual plates, or a combination of the two.14-16 The net pattern of the healing includes a fundamental alteration of the socket volume due to the resorption of the most coronal portion of the alveolar bone (bundle bone), which is functionally connected to the periodontal fibers of the tooth.
With the dental profession now having a better understanding of local micro-anatomy, bone biology, and osseointegration on a cellular and biochemical level than in the past, contemporary practices focus on advancements in bioengineering of metabolically active materials and the creative development of techniques and technology that may decrease postoperative complications, thereby enhancing the predictability of osseointegration, function, and ultimately esthetics.17-21 Ultimately, clinicians are collectively striving for the synthesis of clinically stable osseointegration with maximum hard- and soft-tissue retention. The methodologies used to achieve these endpoints are continually changing as innovative techniques are combined with increased understanding of the anatomical and biological influencers.
Discussion
Vascular Supply
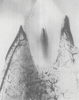
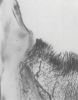
It has been established that the critical vascular supply to the periodontium and supportive alveolar bone is threefold22,23: (1) within the periosteum of the alveolar bone (Figure 1); (2) within the cancellous portion of the alveolar bone proper (Figure 1 and Figure 2); and (3) a vascular plexus within the PDL itself (Figure 2 and Figure 3). This further clarifies the fact that not only does the periodontal functional unit have components that are interdependent to each other, but critical vascular supply to the alveolar bone is composed of three unique units.
Disruption or loss of any one or two of these essential vascular entities would be enough to tip the balance of buccal bone health and survival into atrophy and loss. It is the intraseptal artery contained within the alveolar bone that provides blood supply to the vascular plexus of the PDL. After penetrating the cribriform plate of the socket walls, these vessels create a lattice-like anastomosis encompassing the root structure in a similar manner to a basket.24 This appreciation for the presence of a critical blood supply has also been reviewed as reported observations related to the presence or absence of interdental papillae as a function of the linear distance to the apex of the corresponding interseptal bone.25-27 The authors discussed the critical nature of the blood supply within the PDL to the survival of the interseptal bone, also noting that this blood supply influenced the surrounding soft tissues and their long-term contours.
Besides providing the critical vascular supply contained within, the PDL plays a significant role in the alveolar bone remodeling process. Through the presence or absence of inflammation within the PDL complex, which ultimately may cause up- or down-regulation of pro-inflammatory cytokines and other tissue health-modulating molecules, the RANKL-OPG feedback loop may be influenced. Ultimately, the net effect of this is the ability to promote bone resorption and bone formation by the stimulation of osteoblasts and/or osteoclasts.28 Additionally, the vascular network found within the periodontal ligament has an essential function: that of nutritional delivery of oxygenated hemoglobin, which provides an increased oxygen tension to not only the cells within peri-radicular cellular cementum, but also the inner wall of alveolar bone, thus forming the "lamina dura-periodontal ligament" or "bundle bone" integral complex.29 When tooth loss occurs, the destruction of this complex inevitably ensues, leading to alveolar bone resorption. Since the PDL and the associated "bundle bone," which represents calcified PDL fibers within the buccal wall, are present,9,30 buccal bone loss is noted after tooth extraction.29,31 Cone-beam computed tomography (CBCT) analysis has been used to examine these dimensional alterations that occur in the alveolar process of incisor and premolar sites of the maxilla following tooth removal.9 Results demonstrated that the overall cross-sectional area was reduced from 99.1 mm2 to 65 mm2; height from 11.5 mm to 9.5 mm; and width from 8.5 mm to 3.2 mm (incisal portion), 8.9 mm to 4.8 mm (middle portion), and 9 mm to 5.7 mm (apical portion).
Preventing Bone Loss
Over the past two decades, studies have been conducted to investigate various protocols used to diminish or prevent bone loss after dental extraction. These include immediate implant placement protocols,32,33 soft-tissue augmentation procedures,34,35 palatally biased positioning of the implant within the socket environment,36 the use of platform switching,37,38 and several socket preservation techniques such as "immediate dentoalveolar restoration"39 or the "ice cream" technique,40 among others.41 These techniques have all demonstrated net positive effects on buccal alveolar bone morphology; however, it has been found that over time the negative alterations of the edentulous ridge and/or peri-implant tissues cannot be completely prevented.42-48 Unfortunately, this hard-tissue loss can limit the optimal placement of dental implant(s) and hinder the overall esthetic outcomes of the prosthesis due to alterations in the subcritical and critical peri-implant zones.
Contemporary clinical practices seem to be seeing an increase in patients who require immediate dental implants, primarily within the esthetic zone.49 A fully intact buccal bone wall with a thickness greater than 1 mm and an associated thick gingival phenotype have been noted as the principal anatomical requirements to consider when treatment planning and placing an immediate implant.50 It has been suggested that when both of the aforementioned conditions are present, there is a relatively low risk of recession of the buccal gingiva and width reduction of the soft-tissue profile at the neck of the implant prosthesis.51 Any soft-tissue alterations are biologically suggestive of underlying negative changes (net loss) of the alveolar bone. Caution should be exercised because these conditions are not present in most clinical cases.52 Measurements of the buccal bone wall thickness found that 83% of cases had less than 1 mm of buccal bone thickness while 50% had less than 0.5 mm thickness.53 Other studies have demonstrated that 87% of cases presenting for implant therapy in the anterior maxilla have less than 2 mm of buccal bone thickness, thereby necessitating additional augmentation procedures.54
In an attempt to avoid horizontal and/or vertical alveolar resorption, the preservation of the dental root was first applied via the root submergence concept, aimed at preserving the ridge dimensions for full denture fabrication.55 Subsequent descriptions of this method established definitive metrics by which success was measured, additionally noting the resultant esthetics that occurred upon maintenance of the soft tissues.56-60 Based on this concept, a technique known as "socket shield" was described.61,62 The aim of this technique is to maintain the bundle bone on the buccal side, through a partial root extraction, in order to preserve the crestal bone at the original level. Recently, the concept of partial extraction therapy (PET) was introduced and has been expanded to become an umbrella term used to indicate all partial extraction procedures, ie, socket shield, pontic shield, and root submergence therapy. PET follows the same biological basis as was earlier established with the socket-shield protocol, which remains the most frequently utilized therapeutic intervention of the three available PET procedures.63
Socket Shield
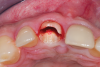
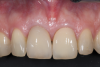
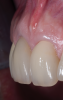
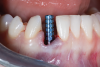
A retrospective evaluation of 128 socket-shield cases in the esthetic zone and posterior sites reported a survival rate of 96.1%. The most common complications noted were internal exposures (exposure of the retained root within the peri-implant sulcus) followed by external exposures (occurring on the external surface of the peri-implant free gingivae) of the retained root shield.64 In reaction to this noted complication, modifications to the original socket-shield technique were subsequently introduced and emphasized a reduced height of the socket-shield remnant to be equal in height to the immediate adjacent bone crest level and then preparation of a chamfer finish to the coronal aspect of the shield's internal dentin surface. This modification was designed to avert the internal exposure of the shield and facilitate fabrication of a prosthetically modified provisional restoration with an "S"-shaped emergence profile thereby enhancing the maximum potential of the resultant soft-tissue infill.65 This modification reduces both internal and external exposures of the socket shields (according to ongoing research by present author HG and colleagues, which is yet unpublished). The technique is depicted in Figure 4 through Figure 10.
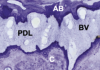
In a 2017 report, human histology, retrieved 5 years post-socket-shield technique, clearly demonstrated the persistent existence of the buccal plate of bone without any deficiency of volume or morphology (Figure 11).66 Furthermore, an intact and healthy PDL was evident and the implant demonstrated osseointegration at the light-microscopic level with a high bone-to-implant contact of 76.2%. The root shield itself did not show any signs of resorption. A critical observation of the author group was that the buccal bone plate was supported and nourished by a healthy, intact PDL. It follows that this nourishment is supplied by the vasculature structures found within the PDL space, thus providing substance to the present authors' thesis that the vascularity within the PDL space is the biologic requisite for socket-shield success.
Recent published data has also examined the volumetric stability of the ridges following the socket-shield technique. Volumetric analysis was used to measure the 3-dimensional stability of the alveolar ridge following immediate implant placement with a socket shield.67 The data showed minimal changes in the buccopalatal dimensions, and it was concluded that the socket-shield technique provided high esthetic outcomes and effective preservation of the facial tissue contours.
Immediate post-extraction implant techniques designed to minimize the volume of tissue loss, which can be considerable, continue to be employed by clinicians.9,33 It is unclear, however, whether or not these protocols effect a net decrease in alveolar bone resorption that occurs post-therapy. Nevertheless, several valid techniques for augmentation of post-extraction sites are utilized and many have been evaluated in randomized controlled trials.68,69 Yet, it is still uncertain if these approaches can consistently reduce horizontal and vertical resorption and whether any could be considered as the premier augmentation technique.70
In a randomized controlled clinical trial in 2011, the researchers compared immediate implant placement with and without bone grafting. Seven out of 74 implants placed failed, and this relatively high rate was attributed also to possible infection of the grafts.71 Subsequently noted, when immediate implant placement was conducted in conjunction with socket-shield preservation utilizing bone substitute grafting in the marginal gap, no infections were reported.72,73 Retention of the buccal aspect of the tooth root for dental implant placement does not appear to interfere with osseointegration, nor does it cause an inflammatory or resorption response.61 Conclusions drawn from these studies suggest that retention of a buccal shield of root structure, comprising dentin, cementum, PDL, and bundle bone, leads to an increase in the stability of the hard and, thus, soft tissues without increased incidence of implant infection. Inference has been drawn that in addition to the retention of dentin, cementum, PDL, and bundle bone, it is the blood supply contained within the PDL that provides the requisite nutrients and oxygen tension to inhibit the resorption of the bundle bone thereby keeping the buccal alveolar bone intact.
Recent evaluation of current evidence on the socket-shield technique has provided data points related to results and complications associated with this technique.74,75 However, both of these reviews, which included not only human but also animal studies and case reports, showed complications after root fragments were unintentionally retained, without restriction of follow-up time for the outcome observations; therefore, there was no demonstratable heterogeneity of data accumulation. Limitations of these reviews include the lack of long-term follow-up and the inadequate definition of the outcomes as analyzed, ultimately leading to non-homogeneous data being included in the reports.
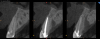
Of note, evaluation of outcome metrics from implant sites performed with socket shields concluded that the socket-shield technique has short-term positive effects related to changes in width and height of buccal bone plate, peri-implant marginal bone levels, esthetic outcomes,76 marginal bone loss reduction, changes in buccal bone width, and higher pink esthetic score (Figure 12 through Figure 14).77-79
In randomized controlled trials, after 6 months of observation, significantly less vertical and horizontal buccal bone resorption and less midfacial mucosal recession was found in a socket-shield group compared to a group comprised of conventional immediate implant placement and provisionalization.80 In a second study, with observations made 1 year following implant restoration, a group with xenograft placed as a gap grafting material demonstrated similar results.81
Conclusion
The maintenance of buccal bone volume in implant therapy continues to be a challenge, as confirmed by the numerous augmentation procedures historically employed. Partial extraction therapy, or socket-shield therapy, appears to be a promising technique that provides the practitioner a single procedure in which the buccal plate of bone and overlying soft-tissue contours can be maintained, resultant 3-dimensional alterations to the alveolar ridge profile minimized, and highly esthetic results achieved. The success of this technique is due largely to retention of the intra-PDL blood supply, which, in turn, promotes maintenance of the peri-implant hard and soft tissues and, consequently, esthetics. More research with larger cohorts is necessary to further validate this technique.
Acknowledgment
The images in Figure 4 through Figure 10 and Figure 12 through Figure 14 were provided by Dr. Gluckman.
About the Authors
Edward D. Karateew, DDS
Clinical Associate Professor and Graduate Program Director, Department of Periodontics, University of Illinois Chicago, Chicago, Illinois
Rodrigo Neiva, DDS, MS
Chairman and Clinical Professor, Department of Periodontics, University of Pennsylvania School of Dental Medicine, Philadelphia, Pennsylvania
Snjezana Pohl, DDS, PhD
Professor, Department of Oral Medicine and Periodontology, University of Rijeka School of Dental Medicine, Rijeka, Croatia
Richard Martin, DDS
Private Practice, Lewisville, Texas
Howard Gluckman, BDS, MChD, PhD
Director, Aesthetic Academy, Cape Town, South Africa; Adjunct Assistant Professor, Department of Periodontics, University of Pennsylvania School of Dental Medicine, Philadelphia, Pennsylvania
Queries to the author regarding this course may be submitted to authorqueries@aegiscomm.com.
References
1. Brånemark PI, Hansson BO, Adell R, et al. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand J Plast Reconstr Surg. 1977;11(suppl 16):1-132.
2. Zarb G, Albrektsson T. Osseointegration: a requiem for the periodontal ligament? Guest editorial. Int J Periodont Rest Dent. 1991;11:88-91.
3. Schroeder A, van der Zypen E, Stich H, Sutter F. The reactions of bone, connective tissue, and epithelium to endosteal implants with titanium-sprayed surfaces. J Maxillofac Surg. 1981;9(1):15-25.
4. Listgarten MA, Lang NP, Schroeder HE, Schroeder A. Periodontal tissues and their counterparts around endosseous implants. Clin Oral Implants Res. 1991;2(3):1-19.
5. Amsterdam M. Periodontal prosthesis. Twenty-five years in retrospect. Alpha Omegan. 1974;67(3):8-52.
6. Araújo MG, da Silva JC, de Mendonca AF, Lindhe J. Ridge alterations following grafting of fresh extraction sockets in man. A randomized clinical trial. Clin Oral Implants Res. 2015;26(4):407-412.
7. Fickl S, Zuhr O, Wachtel H, et al. Dimensional changes of the alveolar ridge contour after different socket preservation techniques. J Clin Periodontol. 2008;35(10):906-913.
8. Schropp L, Wenzel A, Kostopoulos L, Karring T. Bone healing and soft tissue contour changes following single-tooth extraction: a clinical and radiographic 12-month prospective study. Int J Periodontics Restorative Dent. 2003;23(4):313-323.
9. Araújo MG, Lindhe J. Dimensional ridge alterations following tooth extraction. An experimental study in the dog. J Clin Periodontol. 2005;32(2):212-218.
10. Hurng JM, Kurylo MP, Marshall GW, et al. Discontinuities in the human bone-PDL-cementum complex. Biomaterials. 2011;32(29):7106-7117.
11. Amler MH, Johnson PL, Salman I. Histological and histochemical investigation of human alveolar socket healing in undisturbed extraction wounds. J Am Dent Assoc. 1960;61:32-44.
12. Evian CI, Rosenberg ES, Coslet JG, Corn H. The osteogenic activity of bone removed from healing extraction sockets in humans. J Periodontol. 1982;53(2):81-85.
13. Lekovic V, Kenney EB, Weinlaender M, et al. A bone regenerative approach to alveolar ridge maintenance following tooth extraction. Report of 10 cases. J Periodontol. 1997;68(6):563-570.
14. Lang NP, Zitzmann NU, Working Group 3 of the VIII European Workshop on Periodontology. Clinical research in implant dentistry: evaluation of implant-supported restorations, aesthetic and patient-reported outcomes. J Clin Periodontol. 2012;39(suppl 12):133-138.
15. Seibert JS. Reconstruction of deformed, partially edentulous ridges, using full thickness onlay grafts. I. Technique and wound healing. Compend Contin Educ Dent. 1983;4(5):437-453.
16. Wang HL, Al-Shammari K. HVC ridge deficiency classification: a therapeutically oriented classification. Int J Periodontics Restorative Dent. 2002;22(4):335-343.
17. Vercellotti T, Nevins ML, Kim DM, et al. Osseous response following resective therapy with piezosurgery. Int J Periodontics Restorative Dent. 2005;25(6):543-549.
18. Franchi M, Bacchelli B, Giavaresi G, et al. Influence of different implant surfaces on peri-implant osteogenesis: histomorphometric analysis in sheep. J Periodontol. 2007;78(5):879-888.
19. Masuda T, Salvi GE, Offenbacher S, et al. Cell and matrix reactions at titanium implants in surgically prepared rat tibiae. Int J Oral Maxillofac Implants. 1997;12(4):472-485.
20. Qahash M, Hardwick WR, Rohrer MD, et al. Surface-etching enhances titanium implant osseointegration in newly formed (rhBMP-2-induced) and native bone. Int J Oral Maxillofac Implants. 2007;22(3):472-477.
21. Selvakumaran J, Keddie JL, Ewins DJ, Hughes MP. Protein adsorption on materials for recording sites on implantable microelectrodes. J Mater Sci Mater Med. 2008;19(1):143-151.
22. Keller GJ, Cohen DW. India ink perfusions of the vascular plexus of oral tissues. Oral Surg Oral Med Oral Pathol. 1955;8(5):539-542.
23. Bosshardt DD, Bergomi M, Vaglio G, Wiskott A. Regional structural characteristics of bovine periodontal ligament samples and their suitability for biomechanical tests. J Anat. 2008;212(3):319-329.
24. Al-Hezaimi K, Levi P, Rudy R, et al. An extraction socket classification developed using analysis of bone type and blood supply to the buccal bone in monkeys. Int J Periodontics Restorative Dent. 2011;31(4):421-427.
25. Tarnow DP, Magner AW, Fletcher P. The effect of the distance from the contact point to the crest of bone on the presence or absence of the interproximal dental papilla. J Periodontol. 1992;63(12):995-996.
26. Tarnow DP, Cho SC, Wallace SS. The effect of inter-implant distance on the height of inter-implant bone crest. J Periodontol. 2000;71(4):546-549.
27. Tarnow D, Elian N, Fletcher P, et al. Vertical distance from the crest of bone to the height of the interproximal papilla between adjacent implants. J Periodontol. 2003;74(12):1785-1788.
28. Liu J, Zhao Z, Ruan J, et al. Stem cells in the periodontal ligament differentiated into osteogenic, fibrogenic and cementogenic lineages for the regeneration of the periodontal complex. J Dent. 2020;92:103259.
29. Cardaropoli D, Gaveglio L, Gherlone E, Cardaropoli G. Soft tissue contour changes at immediate implants: a randomized controlled clinical study. Int J Periodontics Restorative Dent. 2014;34(5):631-637.
30. Boyne PJ. Osseous repair of the postextraction alveolus in man. Oral Surg Oral Med Oral Pathol. 1966;21(6):805-813.
31. Cardaropoli G, Araújo M, Lindhe J. Dynamics of bone tissue formation in tooth extraction sites. An experimental study in dogs. J Clin Periodontol. 2003;30(9):809-818.
32. Misawa M, Lindhe J, Araújo MG. The alveolar process following single-tooth extraction: a study of maxillary incisor and premolar sites in man. Clin Oral Implants Res. 2016;27(7)884-889.
33. Botticelli D, Persson LG, Lindhe J, Berglundh T. Bone tissue formation adjacent to implants placed in fresh extraction sockets: an experimental study in dogs. Clin Oral Implants Res. 2006;17(4):351-358.
34. Puzio M, Hadzik J, Błaszczyszyn A, et al. Soft tissue augmentation around dental implants with connective tissue graft (CTG) and xenogenic collagen matrix (XCM). 1-year randomized control trial. Ann Anat. 2020;230:151484.
35. Thoma DS, Naenni N, Figuero E, et al. Effects of soft tissue augmentation procedures on peri-implant health or disease: a systematic review and meta-analysis. Clin Oral Implants Res. 2018;29(suppl 15):32-49.
36. Tarnow DP, Chu SJ, Salama MA, et al. Flapless postextraction socket implant placement in the esthetic zone: part 1. The effect of bone grafting and/or provisional restoration on facial-palatal ridge dimensional change - a retrospective cohort study. Int J Periodontics Restorative Dent. 2014;34(3):323-331.
37. Canullo L, Fedele GR, Iannello G, Jepsen S. Platform switching and marginal bone-level alterations: the results of a randomized-controlled trial. Clin Oral Implants Res. 2010;21(1):115-121.
38. Linkevičius T, Puisys A, Steigmann M, et al. Influence of vertical soft tissue thickness on crestal bone changes around implants with platform switching: a comparative clinical study. Clin Implant Dent Relat Res. 2015;17(6):1228-1236.
39. da Rosa JC, de Oliveira Rosa AC, Huwais S. Use of the immediate dentoalveolar restoration technique combined with osseodensification in periodontally compromised extraction sites. Int J Periodontics Restorative Dent. 2019;39(4):527-534.
40. Tan-Chu JH, Tuminelli FJ, Kurtz KS, Tarnow DP. Analysis of buccolingual dimensional changes of the extraction socket using the "ice cream cone" flapless grafting technique. Int J Periodontics Restorative Dent. 2014;34(3):399-403.
41. Heinemann F, Hasan I, Schwahn C, et al. Bone level change of extraction sockets with Bio-Oss collagen and implant placement: a clinical study. Ann Anat. 2012;194(6):508-512.
42. Esposito M, Grusovin MG, Coulthard P, Worthington HV. The efficacy of various bone augmentation procedures for dental implants: a Cochrane systematic review of randomized controlled clinical trials. Int J Oral Maxillofac Implants. 2006;21(5):696-710.
43. Vignoletti F, Matesanz P, Rodrigo D, et al. Surgical protocols for ridge preservation after tooth extraction. A systematic review. Clin Oral Implants Res. 2012;23(suppl 5):22-38.
44. Esposito M, Maghaireh H, Grusovin MG, et al. Soft tissue management for dental implants: what are the most effective techniques? A Cochrane systematic review. Eur J Oral Implantol. 2012;5(3):221-238.
45. Chen ST, Buser D. Esthetic outcomes following immediate and early implant placement in the anterior maxilla - a systematic review. Int J Oral Maxillofac Implants. 2014;29 suppl:186-215.
46. Lin GH, Chan HL, Bashutski JD, et al. The effect of flapless surgery on implant survival and marginal bone level: a systematic review and meta-analysis. J Periodontol. 2014;85(5):e91-e103.
47. Avila-Ortiz G, Elangovan S, Kramer KW, et al. Effect of alveolar ridge preservation after tooth extraction: a systematic review and meta-analysis. J Dent Res. 2014;93(10):950-958.
48. Heinemann F, Hasan I, Bourauel C, et al. Bone stability around dental implants: treatment related factors. Annals of anatomy. Ann Anat. 2015;199:3-8.
49. Evian CI, Waasdorp JA, Ishii M, et al. Evaluating extraction sockets in the esthetic zone for immediate implant placement. Compend Contin Educ Dent. 2011;32(3):e58-e65.
50. Chappuis V, Engel O, Shahim K, et al. Soft tissue alterations in esthetic postextraction sites: a 3-dimensional analysis. J Dent Res. 2015;94(9 suppl):187S-193S.
51. Buser D, Chappuis V, Belser UC, Chen S. Implant placement post extraction in esthetic single tooth sites: when immediate, when early, when late? Periodontol 2000. 2017;73(1):84-102.
52. Januário AL, Duarte WR, Barriviera M, et al. Dimension of the facial bone wall in the anterior maxilla: a cone-beam computed tomography study. Clin Oral Implants Res. 2011;22(10):1168-1171.
53. Gluckman H, Pontes CC, Du Toit J. Radial plane tooth position and bone wall dimensions in the anterior maxilla: a CBCT classification for immediate implant placement. J Prosthet Dent. 2018;120(1):50-56.
54. Huynh-Ba G, Pjetursson BE, Sanz M, et al. Analysis of the socket bone wall dimensions in the upper maxilla in relation to immediate implant placement. Clin Oral Implants Res. 2010;21(1):37-42.
55. Casey DM, Lauciello FR. A review of the submerged-root concept. J Prosthet Dent. 1980;43(2):128-132.
56. Björn H. Free transplantation of gingival propria. Sveriges Tandlak Tidskr. 1963;22:684.
57. Reames RL, Nickel JS, Patterson SS, et al. Clinical, radiographic, and histological study of endodontically treated retained roots to preserve alveolar bone. J Endod. 1975;1(11):367-373.
58. Furhauser R, Florescu D, Benesch T, et al. Evaluation of soft tissue around single-tooth implant crowns: the pink esthetic score. Clin Oral Implants Res. 2005;16(6):639-644.
59. O'Neal RB, Gound T, Levin MP, del Rio CE. Submergence of roots for alveolar bone preservation. I. Endodontically treated roots. Oral Surg Oral Med Oral Pathol. 1978;45(5):803-810.
60. Salama M, Ishikawa T, Salama H, et al. Advantages of the root submergence technique for pontic site development in esthetic implant therapy. Int J Periodontics Restorative Dent. 2007;27(6):521-527.
61. Hürzeler MB, Zuhr O, Schupbach P, et al. The socket-shield technique: a proof-of-principle report. J Clin Periodontol. 2010;37(9):855-862.
62. Siormpas KD, Mitsias ME, Kontsiotou-Siormpa E, et al. Immediate implant placement in the esthetic zone utilizing the "root-membrane" technique: clinical results up to 5 years postloading. Int J Oral Maxillofac Implants. 2014;29(6):1397-1405.
63. Gluckman H, Salama M, Du Toit J. Partial extraction therapies (PET) part 1: maintaining alveolar ridge contour at pontic and immediate implant sites. Int J Periodontics Restorative Dent. 2016;36(5):681-687.
64. Gluckman H, Salama M, Du Toit J. A retrospective evaluation of 128 socket-shield cases in the esthetic zone and posterior sites: partial extraction therapy with up to 4 years follow-up. Clin Implant Dent Relat Res. 2018;20(2):122-129.
65. Gluckman H, Nagy K, Du Toit J. Prosthetic management of implants placed with the socket-shield technique. J Prosthet Dent. 2019;121(4):581-585.
66. Mitsias ME, Siormpas KD, Kotsakis GA, et al. The root membrane technique: human histologic evidence after five years of function. Biomed Res Int. 2017;2017:7269467.
67. Bäumer D, Zuhr O, Rebele S, Hürzeler M. Socket shield technique for immediate implant placement - clinical, radiographic and volumetric data after 5 years. Clin Oral Implants Res. 2017;28(11):1450-1458.
68. Gher ME, Quintero G, Assad D, et al. Bone grafting and guided bone regeneration for immediate dental implants in humans. J Periodontol. 1994;65(9):881-891.
69. Chen ST, Darby IB, Reynolds EC. A prospective clinical study of non-submerged immediate implants: clinical outcomes and esthetic results. Clin Oral Implants Res. 2007;18(5):552-562.
70. Esposito M, Grusovin MG, Polyzos IP, et al. Interventions for replacing missing teeth: dental implants in fresh extraction sockets (immediate, immediate- delayed and delayed implants). Cochrane Database Syst Rev. 2010;(9):CD005968.
71. De Angelis N, Felice P, Pellegrino G, et al. Guided bone regeneration with and without a bone substitute at single post-extractive implants: 1-year post-loading results from a pragmatic multicentre randomised controlled trial. Eur J Oral Implantol. 2011;4(4):313-325.
72. Cherel F, Etienne D. Papilla preservation between two implants: a modified socket-shield technique to maintain the scalloped anatomy? A case report. Quintessence Int. 2014;45(1):23-30.
73. Abitbol J, Antoun H, Degorce T. Implant insertion after tooth extraction: clinical outcomes with different approaches (including socket preservation, immediate, early and delayed placement). Clin Oral Implants Res. 2016;27(suppl 13):530.
74. Gharpure AS, Bhatavadekar NB. Current evidence on the socket-shield technique: a systematic review. J Oral Implantol. 2017;43(5)
:395-403.
75. Mourya A, Mishra SK, Gaddale R, Chowdhary R. Socket-shield technique for implant placement to stabilize the facial gingival and osseous architecture: a systematic review. J Investig Clin Dent. 2019;10
(4):e12449.
76. Atieh MA, Shah M, Abdulkareem M, et al. The socket shield technique for immediate implant placement: a systematic review and meta-analysis. J Esthet Restor Dent. 2021;33(8):1186-1200.
77. Lin X, Gao Y, Ding X, Zheng X. Socket shield technique: a systematic review and meta-analysis. J Prosthodont Res. 2022;66(2):226-235.
78. Velasco Bohórquez P, Rucco R, Zubizarreta-Macho Á, et al. Failure rate, marginal bone loss, and pink esthetic with socket-shield technique for immediate dental implant placement in the esthetic zone. A systematic review and meta-analysis. Biology (Basel). 2021;10(6):549.
79. Sáez-Alcaide LM, González Fernández-Tresguerres F, Cortés-Bretón Brinkmann J, et al. Socket shield technique: a systematic review of human studies. Ann Anat. 2021;238:151779.
80. Abd-Elrahman A, Shaheen M, Askar N, Atef M. Socket shield technique vs conventional immediate implant placement with immediate temporization. Randomized clinical trial. Clin Implant Dent Relat Res. 2020;
22(5):602-611.
81. Atef M, El Barbary A, Dahrous MSE, Zahran AF. Comparison of the soft and hard peri-implant tissue dimensional changes around single immediate implants in the esthetic zone with socket shield technique versus using xenograft: a randomized controlled clinical trial. Clin Implant Dent Relat Res. 2021;23(3):456-465.