You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
Wound healing with regeneration is central to an ideal healing scenario. Biologic surgical additives are agents that supplement local wound healing by virtue of their active components.1 Platelet concentrates have been successfully used as surgical wound additives for more than five decades. The concept of the use of platelet concentrates is to increase the number and, in turn, biologic benefits of platelets at the wound site to provide faster and superior wound healing.
The first published report of a platelet concentrate came from Matras in 1970. This was followed by the first autologous fibrin glues, which eventually paved the way for the development of platelet-rich plasma (PRP).2 The subsequent development of additive-free platelet-rich fibrin (PRF) by Choukroun et al in 2001 announced the arrival of "second-generation" platelet concentrates.3 Since then, a plethora of research and several new platelet concentrates have been proposed. These versions have a variety of differences, most notably in their modes of preparation, forms in which they are obtained, and their application.
The most commonly used second-generation platelet concentrates are L-PRF (leukocyte- and platelet-rich fibrin) and A-PRF (advanced platelet-rich fibrin).4,5 L-PRF was the first proposed high-speed platelet concentrate; A-PRF, based on a slow-speed concept, was introduced later. Both concentrates are widely used in dentistry for applications such as sinus lifts, recession coverage, and management of intrabony or furcation defects. Their usage is owed to their inherent high content of various growth factors such as vascular endothelial growth factor (VEGF), transforming growth factor-β1 (TGF-β1), and platelet-derived growth factor (PDGF).6-8 Both of these concentrates, however, are obtained in a pre-gelled form, which makes them unsuitable for being injected or mixed with biomaterials. Further modification of the original protocol led to the development of liquid PRF. It is obtained in 3 minutes, which is prior to coagulation setting in but is sufficient time for component separation.9 Because coagulation is an active process, once liquid PRF is collected there is a short window of opportunity of around 5 minutes in which the liquid can be either injected or mixed with bone grafts or other biomaterials before it coagulates. This versatility of liquid, or injectable PRF (i-PRF), has led to increased popularity and a broader spectrum of applications. This review provides an update on the protocols, biologic profile, and future applications of liquid PRF, or i-PRF.
Second-Generation Liquid Platelet Concentrates
The original protocol of platelet-rich fibrin resulted in the formation of a pre-clotted, ready-for-use gel. Unlike the first-generation PRP, which was obtained in liquid form and could be mixed with bone grafts, the coagulation process in conventional PRF was already completed.10 This resulted in two major drawbacks: it could not be injected, nor could it be mixed with any biomaterial, such as bone graft, before coagulation. Attempts were made to mix conventional PRF with bone grafts by making pieces of PRF clots and mixing them with bone grafts.11 However, this was just physical mixing, nothing like that achieved by mixing bone graft with PRP, then activating it and having the graft encapsulated in PRP.12 To overcome these limitations, the liquid PRF/liquid fibrinogen protocol was proposed, which suggested the collection of separated components of blood containing platelets and fibrinogen before the initiation of the coagulation process (3 to 8 minutes being the normal amount of time before coagulation sets in).13-16
The first reports in the literature of this kind of liquid concentrate were by Sohn and coworkers followed by Mourão and coworkers in 2015.13,14 It was proposed that a centrifugation of fresh blood be done for 2 minutes at 3300 revolutions per minute (RPM) in noncoated plastic tubes, which lack common clot activators like silica. These authors further demonstrated the concentrate's agglutination with bone graft and noted that it could be used as is or mixed with biomaterials and that it was an excellent autogenous alternative to PRP.
The principal of obtention of liquid concentrate is based on two key factors. First, the tube used is a plain plastic tube without any coatings. A conventional PRF tube is a glass-coated plastic tube with the glass coating containing silica that acts as an activator of coagulation, and, hence, the product is obtained in a clotted form. Plain plastic-coated tubes are inherently hydrophobic and have no coagulation activator, which promotes the concentrate staying in liquid form. The second factor is the short centrifugation time of 2 to 4 minutes. This is so the concentrates are retrieved in liquid form before coagulation sets in, which is normally after 3 to 8 minutes. This time suffices for separation of blood components as per their weight and size. Heavier and larger components such as red blood cells (RBCs) (6 to 8 microns in size) settle at the bottom of the tube and, hence, get excluded from the concentrate, whereas lighter and smaller components such as platelets (2 to 3 microns in size) and leukocytes move toward the top of the tube. The top fraction near RBCs is then aspirated and used as liquid PRF.2,15-17
Effect of Centrifugation Protocols on i-PRF
The original i-PRF protocol was a high-speed, short time protocol using around 3000 RPM.14 Then in 2017, Choukroun, Miron and colleagues, in line with their "slow-speed concept," proposed a modified protocol wherein the centrifugation speed was reduced to 700 RPM for 3 minutes, after which the top yellow part of the tube contents was aspirated and obtained in a liquid form.16 They proposed that the lower speed increases the number of viable cells in the concentrate and may enhance and prolong its biologic activity while still providing enough force and time for efficient separation of cell components. Concurrently, Wend et al performed a detailed study that assessed the effect of varying centrifugation speeds and times. They studied low- (700 RPM), medium- (1400 RPM), and high-speed (2800 RPM) protocols, all for 3 minutes, and observed that lowering the relative centrifugal force resulted in increased numbers of platelets and leukocytes and, consequently, provided more biologic activity in terms of release of various growth factors, such as VEGF, PDGF, and epidermal growth factor (EGF).18
However, in 2020, Miron and colleagues proposed a new concentrated PRF, or C-PRF, protocol where whole blood is centrifuged in uncoated plastic tubes using the high-speed L-PRF protocol, ie, 2700 RPM for 12 minutes.19,20 As per their data, this resulted in a greater separation of blood components, and it was noted that a majority of cells were located right next to the RBC layer in the buffy coat and the top of the tube comprised just platelet-poor plasma. The number of leukocytes in the buffy coat was increased by about 500% to 750% and platelets to about 15 times that of a threefold concentration by the original 700 RPM i-PRF protocol compared to that of blood.20 The authors concluded that selective harvesting of the buffy coat right next to the RBC layer resulted in a more cellular i-PRF at higher speeds.
When different protocols that included 5 minutes, 8 minutes, and 12 minutes at 3000 RPM were studied, it was concluded that the 5-minute protocol was inefficient in causing optimal cell concentration, the 8-minute protocol was satisfactory, and further centrifugation for 12 minutes did not produce any additive effect.20 Also, a much higher release of PDGF-AA, TGF-β1, and EGF and higher cell migration and proliferation of gingival fibroblasts along with increased mRNA production for collagen type I was observed compared with conventional i-PRF.20
The rationale Miron and colleagues provided for such conflicting results from their own previous reports9 that stated that low-speed protocols were superior in production of number of cells, was that the earlier studies assessed i-PRF from the top portion of the tube, which included platelet-poor plasma and may have diluted the i-PRF and/or left behind the most cell-rich portion that is away from the top of the tube just next to the RBC portion. Eventually, they concluded that when the buffy coat next to RBCs was studied, it was abundantly clear that high-speed protocol had superior cellularity and biologic action.19
A vagueness exists in literature over the terminology to be applied for this kind of liquid second-generation platelet concentrate. Additionally, minor modifications of the same protocols have been published with different names.13 There is a need to address this issue to bring about standardization in various research studies. The i-PRF technique is still in its infancy, and over the next few years it is expected that further research will decidedly indicate the superiority of one protocol over another.
Modifications to Original i-PRF Protocol
Several ingenious modifications have been proposed to the original i-PRF protocol. They involve changes in centrifugation speed, amount of centrifugation time, site of aspiration, mixing with other biomaterials to enhance cellularity as well as to obtain composite grafts, and improved in vivo degradation time.
Red i-PRF and Yellow i-PRF
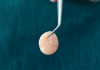
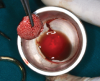
Conventionally, the yellow portion of i-PRF liquid is aspirated and used. However, there are no clear guidelines as to which part of the i-PRF should be aspirated or where exactly the tip of the syringe needle should be placed for aspiration. As per the standard i-PRF protocol, ie, 700 RPM for 3 minutes, based on the level of harvesting i-PRF, the concentrate was classified as either yellow i-PRF or red i-PRF (Figure 1 and Figure 2). Wang et al reported that the harvesting of the upper layer of i-PRF, above the junction of the buffy coat, resulted in yellow i-PRF. Thanasrisuebwong et al, meanwhile, harvested i-PRF from the yellow zone and buffy coat junction, which resulted in red i-PRF.21-23 Upon comparison of red and yellow i-PRF, it was observed that red i-PRF contained more cells and platelet-derived growth factor; however, the yellow i-PRF demonstrated higher fibrin clot formation, α-angle, and clot firmness.22 When the effects of red and yellow i-PRF on human periodontal ligament stem cells were evaluated and compared, it was concluded that red i-PRF better mobilized stem cells and provided for superior migration and proliferation of human periodontal ligament stem cells.22 These enhanced biological properties should result in improved initial wound healing and, thus, may be more efficient when combined with bone grafts for regenerative purposes.
Concentrated-PRF
As noted earlier, Miron and colleagues in 2020 proposed the concept of concentrated PRF, or C-PRF, where whole blood is centrifuged in plain plastic, uncoated test tubes using L-PRF protocol, ie, 2700 RPM for 12 minutes.19,20 This resulted in a greater separation of blood components, and it was noted that most cells in the buffy coat layer of 0.3 ml to 0.5 ml were located right next to the RBC layer. In C-PRF, the yellow portion occupies more than 50% of the tube as compared to around 1 ml to 2 ml (around 10% to 20%) in i-PRF. In C-PRF, the number of leukocytes was increased by about 500% to 750% and platelets to about 15 times that of blood as compared to a threefold concentration of platelets by the original i-PRF protocol.19,20
Albumin PRF
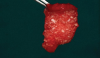
One of the major limitations of conventional PRF is its short in vivo life of around 10 days. To counter this, a novel albumin PRF was recently introduced by Fujioka-Kobayashi et al.24 This was a modification of a similar protocol known as albumin concentrated growth factor, which had an in vivo life of 4 to 6 months, however was completely cell-free.25 The albumin PRF protocol includes centrifugation of 10 ml of blood at 700 g for 8 minutes followed by heating the top platelet-poor plasma portion for 10 mins at 75°C and cooling it to room temperature to create denatured albumin gel. It is then mixed with the buffy coat part (i-PRF) using a female-female Luer lock system (Figure 3). Thus, a simple step of heat-treating PRF is able to prolong the degradation of PRF to around 4 weeks.
Upon ultrastructural examination, it was observed that this preparation had viable cells spread throughout its matrix. Fujioka-Kobayashi et al studied the release of growth factors for 10 days and observed a slow and gradual release of PDGF-AA/BB and TGF-β1.24 Gheno et al studied the biocompatibility and extended degradation properties in comparison with L-PRF, and noted that albumin PRF remained volume stable for 21 days as compared to 7 days for L-PRF.26 This preparation essentially combines the long in vivo life of denatured PRFs with the cellularity and biologic activity of conventional PRF and may be used for guided bone or tissue regeneration. Further studies assessing its cell-occlusive behavior and other properties are required to ascertain its effectiveness in various clinical scenarios.26,27
Addition of Biomaterials
The major advantage of i-PRF over other clotted second-generation platelet concentrates, such as A-PRF and L-PRF, is that it coagulates after it is procured, which allows clinicians to mix in biomaterials that become entrapped in the fibrin matrix. Mixing i-PRF with bone grafts, certain drugs, and other biomaterials has been studied and represents a niche that has huge potential for research and clinical application.
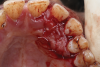
Bone grafts-When Mourão and coworkers first described liquid PRF in 2015, they demonstrated its polymerization, including with bone grafts.14 Bone graft materials still remain the most common component of any composite graft used in combination with i-PRF. Several forms of liquid PRFs, such as autologous fibrin glue (2700 RPM for 2 minutes), concentrated growth factor (accelerated for 30 seconds; centrifuged at 2700 RPM for 2 minutes, 2400 RPM for 4 minutes, 2700 RPM for 4 minutes, and 3000 RPM for 3 minutes; decelerated for 36 seconds to stop), and standard i-PRF (700 RPM for 3 minutes), have been mixed with various grafts to create composite grafts.28-32 In general, once the liquid form of PRF is obtained, it is mixed with particulate graft and left to polymerize. The resultant graft is a 3-dimensional polymerized matrix in which graft particles are encapsulated in a fibrin matrix. This has been referred to as sticky bone or sticky gel (Figure 4 and Figure 5).29,33
Another preparation of bone graft with PRF is known as PRF block. It is a combination of A/L-PRF with the bone graft and i-PRF. In this preparation, PRF and i-PRF are mixed in a ratio of 50:50 with bone graft.34,35 It acts as a biologically active scaffold that not only promotes migration of osteoprogenitor cells but also aids in release of growth factors. These composite grafts have good handling properties and the added advantage of increased volume and have been used for apical defects, intrabony defects, and socket and ridge augmentation.
Antibiotics-Miron and Zhang in a review first pointed out the potential of i-PRF for mixing with antibiotics and then acting as a sustained drug delivery system.36 A pilot study by Rafiee et al assessed combinations of various antimicrobials, including metronidazole, ciprofloxacin, and minocycline, with i-PRF by either immersion or integration. They concluded that immersion was a more effective method and that the drug release was observed in a burst for 24 hours after which a slow release was seen for up to 14 days.37
Horizontal Centrifugation Concept
In 2019, Miron et al proposed a new centrifugation protocol dubbed the gentle centrifugation concept, in which the tube was put in a horizontal position for centrifugation. Superior cell separation and reduced cell damage were observed owing to reduced friction as compared with conventional centrifugation. The authors referred to it as bio-PRF and concluded that it resulted in the highest concentration of platelets and leukocytes.38
Current Evidence Regarding the Applications of i-PRF
Since the inception of i-PRF in 2015, many in vitro, animal, and clinical studies have been done using the basic and other modified protocols. The following sections provide a summary of the current literature (Table 1).21,39-64
Effects on Various Cell Types
A study that assessed the biological effects of liquid PRF and PRP on human periodontal cells demonstrated that i-PRF enhanced cell migration, proliferation, and mineralization compared to PRP. Also, i-PRF reduced the inflammatory mRNA production induced by bacterial lipopolysaccharide.39 This highlights the positive effect of i-PRF on wound healing as well as its immunomodulatory effect. Another comparative in vitro study done on human primary osteoblasts that were incubated with and without i-PRF found that i-PRF incubated cell lines showed enhanced cell proliferation and migration, alkaline phosphatase (ALP) production, bone morphogenetic protein-2 (BMP-2) production, and osteocalcin production.40 This demonstrates potential of i-PRF for faster and superior bone regeneration. In combination with allogenic or xenogenic bone grafts, i-PRF demonstrated enhanced production of BMP-2, ALP, and osteonectin on human osteoblasts,41 indicating that mixing i-PRF with bone grafts enhances biologic activity of the composite.
An in vitro culture model for bone tissue engineering for studying improved angiogenesis was done to assess the effect of i-PRF on outgrowth endothelial cells and primary osteoblasts after 7 days of complex cultivation.42 The study showed the formation of microvessel-like structures, demonstrating improvement of the angiogenesis process. Another study was performed to investigate the biological effects of i-PRF on human dental pulp stem cells. It concluded that 5% of i-PRF enhanced cell migration and proliferation, mRNA for collagen type I alpha 1, dentin sialoprotein, and dentin matrix proteins,43 indicating i-PRF's potential for various endodontic applications. A comparative in vitro study was done using i-PRF and PRP on the gingival stem cells on titanium implant surfaces; it was observed that i-PRF induced higher cell migration as well as high mRNA levels of PDGF, TGF-β, collagen type I, and fibronectin when compared to PRP.21 This demonstrates that i-PRF coating on implant surfaces may lead to superior localized tissue response. Another comparative study, done to substantiate the effects of i-PRF and PRP on the stimulation, migration, proliferation, and collagen synthesis of dermal fibroblasts, showed increased cell migration, proliferation, PDGF mRNA production for TGF-β, and fibronectin with the cell groups cultivated with i-PRF.44
A study to investigate the anti-inflammatory effects of i-PRF on macrophages and dendritic cells found that i-PRF reduced the proinflammatory M1 phenotype of macrophages and activated dendritic cells around infection and also demonstrated inhibition of toll-like receptor-4 (TLR-4) and p-P65.45 A similar study was done on macrophage-like cells to investigate i-PRF's inflammatory response and osteoclastogenesis by the local application of i-PRF.46 The expression interleukin-6 (IL-6) and cyclooxygenase-2were determined, and it was found that i-PRF reduced expression of osteoclast marker genes tartrate-resistant acid phosphatase (TRAP) and cathepsin K.
Antimicrobial Effect
A comparative study was done to evaluate the antimicrobial efficacy of PRP, PRF, and i-PRF by analyzing minimum inhibitory concentration, maximum bactericidal concentration, and biofilm inhibition. The study concluded that all three platelet concentrates have antibacterial effects; however, i-PRF was more active, as a wide zone of inhibition was found against Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans.47 A similar study was done using i-PRF to assess antimicrobial and antibiofilm potential against oral staphylococcus isolates through broth microdilution as a minimum inhibitory concentration. The i-PRF showed antimicrobial efficacy against Staphylococcus aureus and Staphylococcus epidermis.48 These studies demonstrate that i-PRF can be used for infection prevention at surgical sites.
Animal Studies
An experimental animal study was done on 24 Wistar albino rats, with i-PRF injected on the first, third, and seventh days either alone or with scaling and root planing, and tissues were evaluated histopathologically and immunohistochemically. Subgingival injection of i-PRF showed excellent results in reducing bone loss, modulating the inflammatory process, and affecting cytokines.49 A comparative study was done using PRP and i-PRF to evaluate the effect on chondrocytes and osteochondral regeneration on osteochondral defects on rabbit knees. The study was done on 12 adult female New Zealand white rabbits that were sacrificed at 4 and 12 weeks postoperatively and evaluated histologically by macroscopic and microscopic examination for cartilage regeneration. i-PRF was found to have enhanced chondrocyte proliferation and mRNA levels of SOX9, collagen type II, and aggrecan.50
A study was done to compare osteoinductive and osteopromotive variability among different demineralized bone allografts. Twenty-seven male Wistar rats were treated for femoral and intramuscular defects. Histological analysis at 2, 4, and 8 weeks post-implantation, hematoxylin and eosin, safranin-O, TRAP, and osteopontin staining were performed. The study concluded there was significant reduction in inflammatory M1 phenotype of macrophages and activated dendritic cells and inhibition of TLR-4 and p-P65 via the NF-κB pathway.45
Human Clinical Evidence
A randomized controlled trial was conducted to determine whether a connective tissue graft (CTG) combined with i-PRF and a coronally advanced flap (CAF) would improve root coverage of deep Miller Class I or Class II gingival recessions compared to CTG and CAF alone. The CTG was harvested and stored in i-PRF for 15 minutes until a layer of polymerized i-PRF engulfed it. At 6 months postoperatively, complete root coverage was obtained in 88% of sites treated with i-PRF. The study concluded i-PRF with CAF resulted in a superior increase in keratinized tissue and decreased recession depth, which the authors attributed to superior angiogenesis and resistance provided by i-PRF.51 Another study was done to investigate the potential effects of i-PRF on root coverage in free gingival graft (FGG) surgery. Forty patients were randomly divided into two groups; the patients in the control group were treated only with FGG, while the patients in the experiment group were treated with FGG injected with i-PRF as a root surface biomodification agent (FGG + i-PRF). The root coverage obtained was compared after 3 months, and it was shown that FGG + i-PRF significantly improved root coverage.52
A case series was published on CAF combined with sticky bone and i-PRF-coated collagen membrane to treat single maxillary gingival recessions. Patients with isolated Miller Class I or Class II recession in the maxillary esthetic zone were treated using sticky bone (i-PRF + freeze-dried bone allograft [FDBA]) with i-PRF-coated collagen membrane and CAF. Clinical parameters, including probing depth, width of keratinized gingiva, gingival thickness, and recession depth, were recorded at baseline and 6 months post-surgery. The study showed that sticky bone (i-PRF + FDBA) and i-PRF-coated collagen membrane resulted in significantly increased width and thickness of gingiva as well as increased bone thickness.53
A split-mouth study assessed the change in gingival biotype after administration of i-PRF. Ten systemically healthy patients with thin biotype were treated with i-PRF. A statistically significant improvement in the gingival thickness was observed after 1 month of four microneedling injections.54 A comparative study of 36 patients needing FGG looked at the effects of i-PRF on palatal wound healing. Wound healing with hydrogen peroxide test and visual analogue scale for pain was evaluated on the third, seventh, and 14th days and at one month. Groups where i-PRF was used demonstrated improved epithelialization and wound healing, reduced postoperative bleeding, and superior esthetic results.55
In a pilot study that evaluated new bone formation after sinus floor augmentation with collagen plugs impregnated with i-PRF, significant new bone formation was noted at 6 months at both the mesial and distal surfaces of implants. This denotes the role i-PRF may play in implant dentistry.56 A randomized controlled trial evaluated the efficacy of i-PRF in accelerating canine tooth movements and examined the levels of matrix metalloproteinase-8 (MMP-8), IL-1β, RANKL, and osteoprotegerin in the gingival crevicular fluid during orthodontic tooth movement. Twenty patients with Class II division 2 malocclusion were included in the split-mouth study with and without i-PRF. A significant increase in the speed of tooth movement and stimulation in cytokine levels demonstrated the benefits of i-PRF,57 showing its potential in orthodontics.
A randomized controlled trial was done to assess the efficacy of i-PRF on erosive lichen planus. In this split-mouth study one side was treated with i-PRF injection and the other with methylprednisolone acetate in four sessions at 15-day intervals. Injection with i-PRF was found to decrease pain and lesion size and increase satisfaction similar to corticosteroids.58 In a recent case report on the use of i-PRF in the treatment of plasma cell mucositis for a case that was refractory to corticosteroid therapy, infiltrations were performed once a week for 2 months. While total loss of pain and clinically reduced inflammation resulted, complete resolution was not observed.59
Another randomized controlled trial was done for management of dorsal irregularities using rhinoplasty. Forty patients were randomly divided into two groups: with and without i-PRF. The i-PRF was mixed with diced cartilage (producing sticky cartilage). A decrease in the loss of cartilage thickness and increased viability of the cartilage was found in the i-PRF group.60 In a case report on the effect of i-PRF on the treatment of alopecia, patients were given four treatments of i-PRF injections with gaps of 15 days. Although mild bruising was observed for up to 3 days, good improvement in hair growth following injection was seen, which was attributed to the high regenerative potential and presence of stem cells.61 A randomized controlled trial was done to evaluate the benefits of i-PRF in patients with temporomandibular joint (TMJ) pain and dysfunction. Thirty-three patients were randomly selected with TMJ dysfunction, and a significant reduction in pain was seen at 8 weeks, 3 months, 6 months, and 12 months. The study thus concluded that i-PRF had long-term analgesic effects in treatment of painful TMJ derangement and that it would be well-suited for patients with Wilkes stage IV/V (intermediate late to late).62 Another study of i-PRF involving lower facial rejuvenation demonstrated clinically significant benefits in alopecia with i-PRF injections.63
Finally, a case series was published on the effects of i-PRF on dermatological applications, including treatment for alopecia and for enhancement of the periorbital region. The study found that i-PRF had excellent wound healing with favorable growth factor-releasing properties that helped in curing androgenetic alopecia and periorbital rejuvenation, and as temporary filler.64
Discussion and Clinical Applications
The primary difference between the first- and second-generation platelet concentrates is that the latter are completely autologous and free of additives such as calcium chloride and bovine thrombin, which are required for PRP. Introduced to dentistry in 1994, PRP has been widely used for various applications; however, it has had greater prevalence in the medical world where it has been used in treatment of different diseases, such as osteoarthritis, acute muscle injury, rotator cuff repair, tissue regeneration, scar revision, skin rejuvenation, alopecia, and more.65 This usage is commonly attributed to PRP containing 97% platelets, which act as rich sources of PDGF, insulin-like growth factor, VEGF, platelet-derived angiogenic factor, and TGF-β, as well as fibronectin and vitronectin, which are released on activation.65 Upon their introduction to dentistry, second-generation platelet concentrates were quickly accepted for their ease of preparation, excellent biologic activity, and completely autologous nature.5-8 However, most of the medical applications of PRP, such as that of alopecia or tendonitis, were due to its injectable nature, and, thus, conventional PRFs were not so widely used. This is changing, however, because of the recent advancement of a completely additive-free second-generation liquid platelet concentrate.14-17 Based on the authors' clinical experience, i-PRF is now being used with far more frequency than its counterparts, such as A-PRF and L-PRF, for various medical and dental applications.
A limitation of PRP technology is its sudden polymerization upon addition of activators like bovine thrombin and calcium chloride; this is in contrast to the polymerization achieved with i-PRF, which is additive-free and hence physiologic.14 The effect of this is primarily observed in the duration of biologic action; wherein PRP is known to release more than 90% of its growth factor content during the initial 24 hours, PRF has an extended, slow release of up to 7 to 10 days.14-17 This property can be beneficial in extending the biologic action in vivo and may produce superior results. The excellent biologic actions of liquid PRF can be attributed to its 3-dimensional fibrin network embedding platelets, leukocytes, collagen type I, osteocalcin, and various growth factors. These growth factors are slowly released over 10 days and promote enhanced cell migration, proliferation, and mRNA expression of TGF-β, PDGF, collagen type I, and osteocalcin on various cell types, including osteoblasts, chondroblasts, and pulpal cells.14-17
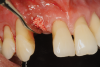
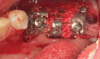
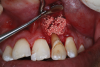
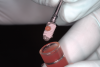
Several desirable properties of i-PRF have prompted its use in dental applications, where it has shown promising results. Such applications include managing Miller Class I and II gingival recessions, improving gingival biotype, bandaging for palatal wound healing, sinus floor augmentation with or without simultaneous implant placement, enhancing the rate of orthodontic tooth movement, aiding lower facial rejuvenation, and treatment of TMJ disorders, erosive oral lichen planus, and plasma cell mucositis, among many others (Figure 6 through Figure 10). Further clinical studies may open up several more applications previously done by PRP, such as management of furcation or intrabony defects in conjunction with bone grafts and implant surface coatings.12 Evidence on cell types, such as periodontal ligament, and gingival fibroblasts, osteoblasts, and chondrocytes on activity such as proliferation and differentiation also indicate potential for cosmetic, dental, and oral regeneration.21,39-45 Another avenue for the use of i-PRF is applications with the addition of biomaterials. When mixed with bone grafts, it forms a pliable block-like mass that has superior properties than conventional particulate and block materials.34,35 Also, the addition of antibiotics may allow for its use in chairside local drug delivery or as an implant coating to achieve superior clinical results.37
Limitations of i-PRF include the fact that it is obtained in small quantities and the short chairside time that it allows for manipulation. However, the quantities obtained are usually enough for most dental applications, and the short time can be managed by quick chairside manipulation.
The research on liquid PRF is in its infancy, as it was first described just about 8 years ago. Its specialty is its liquid nature, which allows it to be manipulated so that certain drugs or biomaterials may be added to its liquid form and incorporated into its polymerized matrix. Currently, the most widely used protocol is 700 RPM for 3 minutes.
Conclusion
Liquid or injectable PRF is a promising platelet concentrate material that shows tremendous potential. Future studies and modifications may pave the way for its increased universal acceptance in medicine as well as dentistry.
Acknowledgment
Photographs courtesy of Bapuji Dental College and Hospital.
About the Authors
Rucha Shah, MDS
Reader, Department of Periodontics, Bapuji Dental College and Hospital,
Karnataka, India
Raison Thomas, MDS
Professor, Department of Periodontics, Bapuji Dental College and Hospital, Karnataka, India
M.G. Triveni, MDS
Professor, Department of Periodontics, Bapuji Dental College and Hospital, Karnataka, India
Baron Tarun Kumar, MDS
Professor, Department of Periodontics, Bapuji Dental College and Hospital, Karnataka, India
Queries to the author regarding this course may be submitted to authorqueries@aegiscomm.com.
References
1. Polimeni G, Xiropaidis AV, Wikesjö UM. Biology and principles of periodontal wound healing/regeneration. Periodontol 2000. 2006;41:30-47.
2. Shah R, M G T, Thomas R, Mehta DS. An update on the protocols and biologic actions of platelet rich fibrin in dentistry. Eur J Prosthodont Restor Dent. 2017;25(2):64-72.
3. Choukroun J, Adda F, Schoeffler C, Vervelle A. Une opportunity en paro-implantologie: le PRF. Implantodontie. 2001;42:55-62.
4. Ghanaati S, Booms P, Orlowska A, et al. Advanced platelet-rich fibrin: a new concept for cell-based tissue engineering by means of inflammatory cells. J Oral Implantol. 2014;40(6):679-689.
5. Dohan DM, Choukroun J, Diss A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I: technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):e37-e44.
6. Choukroun J, Diss A, Simonpieri A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part IV: clinical effects on tissue healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):e56-e60.
7. Dohan DM, Choukroun J, Diss A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part III: leucocyte activation: a new feature for platelet concentrates? Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):e51-e5.
8. Choukroun J, Diss A, Simonpieri A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part V: histologic evaluations of PRF effects on bone allograft maturation in sinus lift. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):299-303.
9. Miron RJ, Fujioka-Kobayashi M, Hernandez M, et al. Injectable platelet rich fibrin (i-PRF): opportunities in regenerative dentistry? Clin Oral Investig. 2017;21(8):2619-2627.
10. Marx RE. Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10(4):225-228.
11. Miron RJ, Moraschini V, Fujioka-Kobayashi M, et al. Use of platelet-rich fibrin for the treatment of periodontal intrabony defects: a systematic review and meta-analysis. Clin Oral Investig. 2021;25(5):2461-2478.
12. Albanese A, Licata ME, Polizzi B, Campisi G. Platelet-rich plasma (PRP) in dental and oral surgery: from the wound healing to bone regeneration. Immun Ageing. 2013;10(1):23.
13. Sohn DS, Huang B, Kim J, et al. Utilization of autologous concentrated growth factors (CGF) enriched bone graft matrix (sticky bone) and CGF-enriched fibrin membrane in implant dentistry. J Implant Adv Clin Dent. 2015;7:11-29.
14. Mourão CF, Valiense H, Melo ER, et al. Obtention of injectable platelets rich-fibrin (i-PRF) and its polymerization with bone graft: technical note [in English, Portuguese]. Rev Col Bras Cir. 2015;42(6):421-423.
15. Varela HA, Souza JCM, Nascimento RM, et al. Injectable platelet rich fibrin: cell content, morphological, and protein characterization. Clin Oral Investig. 2019;23(3):1309-1318.
16. Fujioka-Kobayashi M, Miron RJ, Hernandez M, et al. Optimized platelet-rich fibrin with the low-speed concept: growth factor release, biocompatibility, and cellular response. J Periodontol. 2017;88(1):112-121.
17. Choukroun J, Ghanaati S. Reduction of relative centrifugation force within injectable platelet-rich-fibrin (PRF) concentrates advances patients' own inflammatory cells, platelets and growth factors: the first introduction to the low speed centrifugation concept. Eur J Trauma Emerg Surg. 2018;44(1):87-95.
18. Wend S, Kubesch A, Orlowska A, et al. Reduction of the relative centrifugal force influences cell number and growth factor release within injectable PRF-based matrices. J Mater Sci Mater Med. 2017;28(12):188.
19. Miron RJ, Chai J, Zhang P, et al. A novel method for harvesting concentrated platelet-rich fibrin (C-PRF) with a 10-fold increase in platelet and leukocyte yields. Clin Oral Investig. 2020;24(8):2819-2828.
20. Fujioka-Kobayashi M, Katagiri H, Kono M, et al. Improved growth factor delivery and cellular activity using concentrated platelet-rich fibrin (C-PRF) when compared with traditional injectable (i-PRF) protocols. Clin Oral Investig. 2020;24(12):4373-4383.
21. Wang X, Zhang Y, Choukroun J, et al. Behavior of gingival fibroblasts on titanium implant surfaces in combination with either injectable-PRF or PRP. Int J Mol Sci. 2017;18(2):331.
22. Thanasrisuebwong P, Kiattavorncharoen S, Surarit R, et al. Red and yellow injectable platelet-rich fibrin demonstrated differential effects on periodontal ligament stem cell proliferation, migration, and osteogenic differentiation. Int J Mol Sci. 2020;21(14):5153.
23. Thanasrisuebwong P, Surarit R, Bencharit S, Ruangsawasdi N. Influence of fractionation methods on physical and biological properties of injectable platelet-rich fibrin: an exploratory study. Int J Mol Sci. 2019;20(7):1657.
24. Fujioka-Kobayashi M, Schaller B, Mourão CF, et al. Biological characterization of an injectable platelet-rich fibrin mixture consisting of autologous albumin gel and liquid platelet-rich fibrin (Alb-PRF). Platelets. 2021;32(1)74-81.
25. Barros Mourão CF, Gheno E, Lourenço ES, et al. Characterization of a new membrane from concentrated growth factors associated with denaturized Albumin (Alb-CGF) for clinical applications: a preliminary study. Int J Growth Factors Stem Cells Dent. 2018;1(2):64-69.
26. Gheno E, Barros Mourão CF, de Mello-Machado RC, et al. In vivo evaluation of the biocompatibility and biodegradation of a new denatured plasma membrane combined with liquid PRF (Alb-PRF). Platelets. 2021;32(4):542-554.
27. Kargarpour Z, Nasirzade J, Panahipour L, et al. Liquid platelet-rich fibrin and heat-coagulated albumin gel: bioassays for TGF-β activity. Materials (Basel). 2020;13(16):3466.
28. Chandra RV, Shivateja K, Reddy AA. Autogenous bone ring transplant vs autologous growth factor-enriched bone graft matrix in extraction sockets with deficient buccal bone: a comparative clinical study. Int J Oral Maxillofac Implants. 2019;34(6):1424-1433.
29. Joshi CP, D'Lima CB, Karde PA, Mamajiwala AS. Ridge augmentation using sticky bone: a combination of human tooth allograft and autologous fibrin glue. J Indian Soc Periodontol. 2019;23(5):493-496.
30. Sureshbabu NM, Ranganath A, Jacob B. Concentrated growth factor - surgical management of large periapical lesion using a novel platelet concentrate in combination with bone graft. Ann Maxillofac Surg. 2020;10(1):246-250.
31. Soni R, Priya A, Yadav H, et al. Bone augmentation with sticky bone and platelet-rich fibrin by ridge-split technique and nasal floor engagement for immediate loading of dental implant after extracting impacted canine. Natl J Maxillofac Surg. 2019;10(1):98-101.
32. Shah R, Gowda TM, Thomas R, et al. Biological activation of bone grafts using injectable platelet-rich fibrin. J Prosthet Dent. 2019;121(3):391-393.
33. Shukla S, Chug A, Mahesh L, et al. Optimal management of intrabony defects: current insights. Clin Cosmet Investig Dent. 2019;11:19-25.
34. Cortellini S, Castro AB, Temmerman A, et al. Leucocyte- and platelet-rich fibrin block for bone augmentation procedure: a proof-of-concept study. J Clin Periodontol. 2018;45(5):624-634.
35. Castro AB, Cortellini S, Temmerman A, Li X, Pinto N, Teughels W, Quirynen M. Characterization of the leukocyte- and platelet-rich fibrin block: release of growth factors, cellular content, and structure. Int J Oral Maxillofac Implants. 2019;34(4):855-864.
36. Miron RJ, Zhang Y. Autologous liquid platelet rich fibrin: a novel drug delivery system. Acta Biomater. 2018;75:35-51.
37. Rafiee A, Memarpour M, Taghvamanesh S, et al. Drug delivery assessment of a novel triple antibiotic-eluting injectable platelet-rich fibrin scaffold: an in vitro study. Curr Pharm Biotechnol. 2021;22(3):380-388.
38. Miron RJ, Chai J, Zheng S, et al. A novel method for evaluating and quantifying cell types in platelet rich fibrin and an introduction to horizontal centrifugation. J Biomed Mater Res A. 2019;107(10):2257-2271.
39. Zheng S, Zhang X, Zhao Q, et al. Liquid platelet-rich fibrin promotes the regenerative potential of human periodontal ligament cells. Oral Dis. 2020;26(8):1755-1763.
40. Wang X, Zhang Y, Choukroun J, et al. Effects of an injectable platelet-rich fibrin on osteoblast behavior and bone tissue formation in comparison to platelet-rich plasma. Platelets. 2018;29(1):48-55.
41. Kyyak S, Blatt S, Pabst A, et al. Combination of an allogenic and a xenogenic bone substitute material with injectable platelet-rich fibrin - a comparative in vitro study. J Biomater Appl. 2020;35(1):83-96.
42. Dohle E, El Bagdadi K, Sader R, et al. Platelet-rich fibrin-based matrices to improve angiogenesis in an in vitro co-culture model for bone tissue engineering. J Tissue Eng Regen Med. 2018;12(3):598-610.
43. Chai J, Jin R, Yuan G, et al. Effect of liquid platelet-rich fibrin and platelet-rich plasma on the regenerative potential of dental pulp cells cultured under inflammatory conditions: a comparative analysis. J Endod. 2019;45(8):1000-1008.
44. Wang X, Yang Y, Zhang Y, Miron RJ. Fluid platelet-rich fibrin stimulates greater dermal skin fibroblast cell migration, proliferation, and collagen synthesis when compared to platelet-rich plasma. J Cosmet Dermatol. 2019;18(6):2004-2010.
45. Zhang J, Yin C, Zhao Q, et al. Anti-inflammation effects of injectable platelet-rich fibrin via macrophages and dendritic cells. J Biomed Mater Res A. 2020;108(1):61-68.
46. Kargarpour Z, Nasirzade J, Panahipour L, et al. Liquid PRF reduces the inflammatory response and osteoclastogenesis in murine macrophages. Front Immunol. 2021;12:636427.
47. Kour P, Pudakalkatti PS, Vas AM, et al. Comparative evaluation of antimicrobial efficacy of platelet-rich plasma, platelet-rich fibrin, and injectable platelet-rich fibrin on the standard strains of Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans. Contemp Clin Dent. 2018;9(suppl 2):S325-S330.
48. Jasmine S, A T, Janarthanan K, et al. Antimicrobial and antibiofilm potential of injectable platelet rich fibrin - a second-generation platelet concentrate-against biofilm producing oral staphylococcus isolates. Saudi J Biol Sci. 2020;27(1):41-46.
49. Aydinyurt HS, Sancak T, Taskin C, et al. Effects of injectable platelet-rich fibrin in experimental periodontitis in rats. Odontology. 2021;109(2):422-432.
50. El Raouf MA, Wang X, Miusi S, et al. Injectable-platelet rich fibrin using the low speed centrifugation concept improves cartilage regeneration when compared to platelet-rich plasma. Platelets. 2019;30(2):213-221.
51. Turer U, Ozcan M, Alkaya B, et al. Clinical evaluation of injectable platelet-rich fibrin with connective tissue graft for the treatment of deep gingival recession defects: a controlled randomized clinical trial. J Clin Periodontol. 2020;47(1):72-80.
52. İzol BS, Üner DD. A new approach for root surface biomodification using injectable platelet-rich fibrin (I-PRF). Med Sci Monit. 2019;25:4744-4750.
53. Kapa BP, N K S, G V G, Mehta DS. Coronally advanced flap combined with sticky bone and i-PRF-coated collagen membrane to treat single maxillary gingival recessions: case series. Clin Adv Periodontics. 2021; doi: 10.1002/cap.10164.
54. Ozsagir ZB, Saglam E, Yilmaz BS, et al. Injectable platelet-rich fibrin and microneedling for gingival augmentation in thin periodontal phenotype: a randomized controlled clinical trial. J Clin Periodontol. 2020;47(4):489-499.
55. Kızıltoprak M, Uslu MÖ. Comparison of the effects of injectable platelet-rich fibrin and autologous fibrin glue applications on palatal wound healing: a randomized controlled clinical trial. Clin Oral Investig. 2020;24(12):4549-4561.
56. Gülşen U, Dereci Ö. Evaluation of new bone formation in sinus floor augmentation with injectable platelet-rich fibrin-soaked collagen plug: a pilot study. Implant Dent. 2019;28(3):220-225.
57. Erdur EA, Karakaslı K, Oncu E, et al. Effect of injectable platelet-rich fibrin (i-PRF) on the rate of tooth movement. Angle Orthod. 2021;91(3):285-292.
58. Saglam E, Ozsagir ZB, Unver T, et al. Efficacy of injectable platelet-rich fibrin in the erosive oral lichen planus: a split-mouth, randomized, controlled clinical trial. J Appl Oral Sci. 2021;29:e20210180.
59. Gasparro R, Adamo D, Masucci M, et al. Use of injectable platelet-rich fibrin in the treatment of plasma cell mucositis of the oral cavity refractory to corticosteroid therapy: a case report. Dermatol Ther. 2019;32(5):e13062.
60. Gode S, Ozturk A, Berber V, Kısmalı E. Effect of injectable platelet-rich fibrin on diced cartilage's viability in rhinoplasty. Facial Plast Surg. 2019;35(4):393-396.
61. Arora R, Shukla S. Injectable-platelet-rich fibrin-smart blood with stem cells for the treatment of alopecia: a report of three patients. Int J Trichology. 2019;11(3):128-131.
62. Albilia J, Herrera-Vizcaino C, Weisleder H, et al. Liquid platelet-rich fibrin injections as a treatment adjunct for painful temporomandibular joints: preliminary results. Cranio. 2020;38(5):292-304.
63. Nacopoulos C, Vesala AM. Lower facial regeneration with a combination of platelet-rich fibrin liquid matrices based on the low speed centrifugation concept - Cleopatra technique. J Cosmet Dermatol. 2020;19(1):185-189.
64. Shashank B, Bhushan M. Injectable platelet-rich fibrin (PRF): the newest biomaterial and its use in various dermatological conditions in our practice: a case series. J Cosmet Dermatol. 2021;20(5):1421-1426.
65. Martínez-Martínez A, Ruiz-Santiago F, García-Espinosa J. Platelet-rich plasma: myth or reality? Radiologia (Engl Ed). 2018;60(6):465-475.