You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
Drug-induced gingival enlargement (DIGE) is a multifactorial gingival condition associated with the use of calcium channel blockers (CCBs), immunosuppressants, and anticonvulsants. A variety of therapies, including nonsurgical, surgical, and maintenance, are used to manage patients with DIGE. Ultimately, the goal of DIGE treatment is to re-establish a healthy, maintainable periodontium and interrupt the positive feedback cycle that results in persistent gingival enlargement.
Epidemiology
DIGE is a plaque-induced, multifactorial, gingival inflammatory condition mediated by pharmacological agents.1 The term "drug-induced gingival enlargement," or "overgrowth," has superseded the terms "hyperplasia" and "hypertrophy," as these previously labeled the disease with an assumed pathogenicity.2 Gingival enlargement is defined as an increase in the size of the keratinized gingiva. In DIGE, gingival overgrowth is the result of inducing-drug use, which becomes exacerbated in the presence of dental biofilm. The prevalence of DIGE has been reportedly as high as 50%3; however, several risk factors play a role in its epidemiology. Furthermore, DIGE varies with the classes of drugs that are known to cause gingival enlargement, including mainly antihypertensive, immunosuppressive, and anticonvulsant medications.
DIGE makes oral hygiene maintenance cumbersome, and the task of maintaining oral hygiene becomes increasingly challenging as the disease progresses (Figure 1). Implications of this may inevitably lead to poorer periodontal and oral health outcomes. Additionally, gingival pain and masticatory dysfunction may become prominent as DIGE worsens. In cases of severe overgrowth, permanent gingival disfigurations may occur.4 Therefore, dental professionals should be well-versed in the identification and management of DIGE to optimize patient care and avoid such unfavorable outcomes.
Pathophysiology
Tungare and Paranjpe recently described advancements in the pathophysiology of DIGE.5 Research suggests there may be a genetic component to DIGE due to its associated interpatient variability6; however, the nature of the disease is greatly multifactorial. From a histopathological perspective, the environment responsible for facilitating the pathophysiology of DIGE is the connective tissue (CT). The CT houses gingival fibroblasts and matrix metalloproteinases (MMPs), both of which play a significant role in the onset of DIGE.7 It has been postulated that certain fibroblast phenotypes influenced by human lymphocyte antigens (HLA) may result in genetic predisposition to DIGE.7 Furthermore, MMPs and their counterparts, tissue inhibitors of metalloproteinases, regulate collagen synthesis. In the presence of an inducing drug, the homeostasis of calcium influx is disrupted. As a result, folic acid uptake is reduced, which downregulates the degradation of collagen by active MMP-1.7,8 This results in excessive collagen deposition and, consequently, enlargement of the gingiva.
Biofilm-induced gingival inflammation further contributes to the pathogenesis of DIGE. Specifically, cytokines play an important role. The pro-inflammatory markers interleukin-1 beta (IL-1β) and IL-6 are expressed during acute gingival inflammation and involved in fibroblastic processes, such as the production of ground substance.5 In the presence of an inducing drug, HLA-influenced fibroblasts proliferate, resulting in excessive buildup of collagen and glycosaminoglycans, thereby increasing the CT volume.5,9,10
Risk Factors
The major risk factors for DIGE include age, sex, genetics, drug and periodontal variables, and concomitant medication.7,11 In general, DIGE tends to affect older individuals with a male predilection. The relationship between drug dose and the onset of DIGE remains unclear for some drugs, like nifedipine8,12; however, this correlation has been proposed to exist for other medications, such as amlodipine.13-15 Nonetheless, a positive correlation between increasing dose and DIGE has yet to be established for all inducing drugs. Concurrent use of two or more inducing drugs significantly increases the risk for DIGE, especially cyclosporine and CCBs when used in the treatment of organ transplant patients.11 In cases of combination therapy, it may be difficult to determine which drug is primarily attributed to DIGE. Therefore, consultation with the patient's family physician is necessary to narrow down the specific culprit. This may be achieved by either discontinuing one drug at a time, under the physician's discretion, or perhaps investigating any recent changes in drug variables such as increased dosage.16
Dental plaque plays a clinically significant role in the onset of DIGE, yet insufficient evidence exists to characterize it as a direct risk factor. Nonetheless, it has been well-documented that dental plaque acts as a cofactor in the etiology of DIGE.4 In the 2017 classification system for periodontal diseases and conditions, plaque is identified as an essential factor to stimulate and exacerbate acute gingival inflammation; however, it was not recognized as a universal risk factor for DIGE.1 Recent studies suggest dental biofilm may be a modifiable risk factor for certain types of gingival enlargement. A systematic review highlights that poor plaque control was the most common risk factor for the development of amlodipine-associated gingival enlargement.17 Ultimately, the role of dental plaque may vary in the onset of DIGE. Still, dental plaque and poor oral hygiene are well-correlated with disease severity and, therefore, must be addressed.
Drugs Associated With Gingival Enlargement
Historically, three major categories of systemic drugs have been reported to induce gingival enlargement: CCBs, immunosuppressants, and anticonvulsants (Table 1).18 In addition to this classical triad of inducing drugs, cannabis derivatives have been potentially linked to DIGE.
Calcium Channel Blockers
CCBs are mainly used in the treatment of cardiovascular conditions, including hypertension, peripheral vascular disease, and angina pectoris. Examples of CCBs include nifedipine, amlodipine, verapamil, and diltiazem (Table 1). Of all the CCBs, nifedipine (Adalat®, Afeditab®) is most frequently implicated with DIGE. The gingival effects of nifedipine were first reported by Lederman et al in 1984,19 and since then several follow-up studies have supported its association. The prevalence of nifedipine-induced gingival enlargement ranges from 14% to 83%, whereas the prevalence of DIGE for verapamil (Calan®, Verelan®) and amlodipine (Katerzia®, Norvasc®) is 4.2% and 3.3%, respectively.7
Patients taking nifedipine are at a significantly higher risk of developing gingival overgrowth compared to those taking amlodipine or diltiazem (Cardizem®, Tiazac®).7,14,20 Nifedipine is highly lipophilic, which allows it to penetrate cells with relative ease, whereas amlodipine requires a complex transport mechanism to enter cells due to its polarity.7 Furthermore, amlodipine has a higher volume of distribution than nifedipine; therefore, it circulates in the blood at lower concentrations. Conversely, nifedipine maintains a higher plasma concentration that enables it to surpass the threshold required to initiate gingival changes more effectively.7
On their own, CCBs are known to induce gingival enlargement; however, when combined with other inducing drugs, they may have a synergistic effect to potentiate DIGE. For example, nifedipine and diltiazem are known to exacerbate DIGE in immunosuppressive patients who are taking cyclosporine.5
Immunosuppressants
Immunosuppressant drugs are most commonly indicated to reduce the risk of infection following solid organ transplants and to treat patients who are immunocompromised. Cyclosporine, sirolimus, and tacrolimus are common pharmacotherapies for these patients and are known to induce DIGE (Table 1).
With up to 70% incidence, cyclosporine (Gengraf®, Neoral®) is the most frequently associated immunosuppressant with DIGE.21 Specifically, it has shown to induce DIGE in up to 53% of renal transplant patients.22 Cyclosporine-initiated gingival overgrowth may be influenced by the susceptibility of gingival fibroblasts to interact with cyclosporine and its metabolites.23 It has also been postulated that plaque-induced gingival inflammation can exacerbate these interactions, thereby potentiating the disease.23 While further studies are required to confirm this, it is valuable for clinicians to identify the potential for crosstalk between these two risk factors.
Tacrolimus (Advagraf®, Prograf®) is an immunosuppressant typically used to prevent graft rejection for organ transplant patients. In comparison to cyclosporine, research suggests tacrolimus may have less systemic adverse effects, including reduced hepatotoxicity and nephrotoxicity; however, this remains controversial.24 Nonetheless, tacrolimus has fewer reported negative outcomes in terms of the extent and severity of DIGE.25 In fact, a study by James et al reported a better prognosis for patients experiencing gingival overgrowth over a period of 6 to 9 months following the replacement of cyclosporine with tacrolimus.26
Immunosuppressants as Anti-COVID-19 Agents
Considering the current pandemic, it is important to mention the role of immunosuppressants within the realm of COVID-19. Immunosuppressants, in general, have been suggested to potentially inhibit the antibody response produced by COVID-19 vaccines.27 Cyclosporine in particular has been studied for this purpose, as well as for its role as a possible therapeutic substitute for first-line COVID-19 therapy.27-30 Research suggests cyclosporine therapy is associated with a reduction in antibody titers post-vaccination for influenza, keyhole limpet, hemocyanin, tetanus, and hepatitis B virus.28-30 However, the effect of cyclosporine on COVID-19 vaccinations, including adenovirus vectors (AstraZeneca Canada Inc, Janssen Inc [Johnson & Johnson Canada]), inactivated virus, and mRNA vaccines (Pfizer-BioNTech Manufacturing GmbH, Moderna Therapeutics Inc) is yet to be determined.
Recent studies have identified cyclosporine as a possible treatment modality for COVID-19.31 Cyclosporine has demonstrated anti-SARS-CoV-2 antiviral activity and may therefore play a valuable role as first-line therapy for COVID-19.31 The repurposing of drugs with anti-inflammatory and antiviral properties, such as cyclosporine, is an essential step in finding a safe and effective treatment for COVID-19. Although recent studies have elucidated this, further research is required to assess the benefit/risk ratio of using cyclosporine for anti-COVID-19 therapy.31 Also, given the well-documented association between cyclosporine and DIGE, patients with COVID-19 who are treated with cyclosporine must be monitored carefully for the development of DIGE, and the appropriate interventions should be put in place when necessary.
Anticonvulsants
Anticonvulsants have also been documented to induce DIGE, especially in patients with polypharmacy regimens.5 The primary use of such medications is to prevent epileptic episodes. These drugs include phenytoin, sodium valproate, phenobarbital, and ethosuximide.
Phenytoin is the most commonly associated antiepileptic medication with DIGE. It is primarily used to treat temporal lobe, grand mal, and psychomotor seizures.3 Furthermore, it has been used in the treatment of chronic neuropathic pain and fibromyalgia.32 The incidence of phenytoin-induced gingival enlargement ranges from 3% to 93%; however, patients on long-term regimens are more vulnerable to developing DIGE.33 Phenytoin is known to accumulate in the brain at levels tenfold higher than its serum concentrations,34 yet circulating levels of phenytoin have been considered a risk factor for DIGE.35 Interestingly, plasma levels seem to play a more significant role in the onset of DIGE, while its concentration in gingival crevicular fluids is less influential.35
Valproic acid is a rare inducer of gingival enlargement. The main factors that influence its adverse activity include genetic predisposition, drug variables (ie, dose), and biofilm-mediated inflammatory changes to gingival tissues.36 Although DIGE tends to occur in older males, this does not necessarily apply to sodium valproate. Albeit rare, sodium valproate has been shown to induce DIGE in a 22-month-old child suffering from epilepsy.37 Therefore, age may not be the best predictor of DIGE, especially for patients on antiepileptic therapies.
Cannabis as a Potential Inducing Drug
Cannabis has been identified as a potential inducing drug.18 Cannabidiol, a cannabis extract, is structurally similar to phenytoin and has been demonstrated in vitro to promote a tenfold increase in fibroblastic activity while downregulating MMPs at higher concentrations.38 Recently documented case reports also imply a possible association between marijuana and DIGE, especially in combination with tobacco use.38-40 While these studies identify that a link between cannabis use and DIGE may exist, the quality of evidence is insufficient. As such, rigorous double-blind randomized clinical trials are required to confirm the association between cannabis use and DIGE.
Clinical Presentation
DIGE becomes clinically discernible within 2 to 4 months after the onset of pharmacotherapy with an inducing drug.3,16 The early stages of DIGE may present as painless, beadlike enlargements of the interdental papillae that extend to the facial and lingual gingival margins.16 As the disease progresses, and in the absence of inflammation, DIGE can be described as mulberry-shaped, firm, pale, pink, and resilient, with a minimally lobulated surface that tends not to bleed upon probing.41 A tissue fold covering a large portion of the crown can form along with the progression and unification of the marginal and papillary gingivae.41 This most often appears as an enlargement beneath the gingival margin, which is separated by a linear groove.41
This classical presentation of DIGE may vary significantly depending on local and systemic risk factors and patient-specific variables.7 Local risk factors such as calculus and malpositioned teeth increase biofilm retention. As gingival enlargement worsens, plaque control may become increasingly difficult, as noted earlier. With increased plaque build-up, acute gingival inflammation may not resolve and can exacerbate DIGE. When this happens, the color of the gingiva transitions to bluish-red, an increase in bleeding on probing (BOP) occurs, and the lobulated surface demarcations are obliterated.41
DIGE tends to occur exclusively on the attached gingiva of the maxillary and mandibular alveolar processes where teeth are present. Specifically, it is most pronounced on the facial/buccal surfaces of the anterior dentition.7 Extraction of nonrestorable teeth may result in DIGE resolution and re-establishment of a healthy gingival architecture; however, in rare occasions DIGE may not entirely resolve. Completely edentulous patients may also experience gingival enlargement, especially if their oral hygiene is poor and they have a history of DIGE. Additionally, patients with titanium dental implants may be at risk for DIGE, since biofilm accumulation is possible in the peri-implant sulcus.42-44 Although these instances may occur, DIGE of the edentulous and peri-implant mucosae is rare.42,43
Differential Diagnoses
Several periodontal inflammatory conditions and systemic diseases manifest similarly to DIGE in terms of their clinical appearances (Table 2).5,16,41 It is imperative to differentiate between DIGE and its clinical counterparts as treatment strategies may vary.16 Gingival enlargements are not specific only to drug use; that is, many periodontal diseases and conditions may demonstrate gingival enlargement.45 As such, a comprehensive periodontal evaluation, including radiographic examination, must be conducted to rule out synchronal periodontal inflammatory diseases, namely, gingivitis or periodontitis.1,5 There is a paucity of research regarding the coexistence of DIGE with these inflammatory conditions. Nonetheless, scaling and root planing (SRP) should be performed with a subsequent periodontal re-evaluation for patients who may be experiencing periodontitis concurrently with DIGE.
Clinically, the size of the gingival tissues in DIGE should exceed those in edema purely from an acute inflammatory response, especially in the absence of an inducing drug.1 However, these differences can be extremely subtle, potentially posing challenges for strictly diagnosing based on clinical appearance. Where the clinical presentation of gingival enlargement is unusual, such as in the presence of erythroleukoplakia, an irregular nodular pattern, or purulent exudate, then histopathological investigation may be required to rule out diagnoses that are concerning.46,47 This is particularly important for persisting or recurrent gingival overgrowths, which may indicate malignant changes, such as those seen with oral squamous cell carcinoma.48 As such, for these types of serious lesions an incisional biopsy may be warranted.
Systemic diseases and conditions that cause generalized gingival enlargement, including scurvy and leukemia, also fall under the differential diagnoses for DIGE (Table 2).49 Unlike DIGE, however, these systemic diseases will generally present with BOP. Although scurvy is infrequently encountered in developed nations, it may be prudent to consider a nutritional analysis and/or hematological testing to determine vitamin C plasma levels for patients who are at risk. Furthermore, a complete blood-cell count (CBC) should be considered in cases where gingival enlargement is accompanied by profuse bleeding to rule out concurrent conditions, including anemia and/or leukemia.5 Lastly, a culture sample may be obtained to exclude additional differential diagnoses, such as pseudomembranous candidiasis.5
Ultimately, the gingivae are live tissues that can be affected by a multitude of factors to cause enlargement. It is the responsibility of the dental professional to identify any variation from normal as early as possible and to assess which tests should be performed to reach an accurate final diagnosis. Emphasis should be placed on the basic nature of the disease to be able to treat it effectively. To date, although variants of gingival inflammation can be identified, what cannot be accurately predicted is which of these variants will progress into destructive periodontal diseases, such as periodontitis.1 Therefore, it is important to intervene as early as possible to try to preserve the integrity of the periodontium.
Diagnosis and Classification
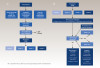
To reach a clinical diagnosis of DIGE, the clinician must first conduct a thorough review of the patient's history to identify any potential risk factors (Figure 2). DIGE should always be included near the top of the differential diagnosis for patients who present with known risk factors, experience gingival enlargement, and concurrently take an inducing drug. As mentioned earlier, further testing may be required to rule out other diseases and conditions on the list of differential diagnoses (Table 2). This is highly dependent on the individual and based on a combination of medical history, periodontal and radiographic examinations, and clinical presentation. Once a diagnosis of DIGE is confirmed, it must be graded in order to guide the subsequent treatment (Table 3).50
Murakami et al summarized the classification of generalized gingival enlargement as either mild, moderate, or severe, with the progressive involvement of the gingival papillae, marginal gingiva, and attached gingiva, respectively.1 A myriad of additional methods and clinical indices have also been proposed to quantify the severity of DIGE.51 Specifically, these indices assess the vertical and horizontal components of gingival overgrowth with a score, or grade, to systematically guide treatment strategies. Early stages of DIGE may present with horizontal growth, as indicated by nodular enlargement of the interdental papillae in a buccolingual dimension.52 As DIGE increases in severity, vertical growth extending toward the dental crown becomes more prominent.
Perhaps the most relevant clinical index used to assess the severity of DIGE and guide standardized treatment recommendations was proposed by Ingles et al in 1999 (Table 3).50 This index is a modification of a previously described index by Seymour et al53 with recommendations for appropriate surgical intervention. The Ingles index grades the severity of DIGE from 0 to 4, where grade 3 marks the threshold recommended to implement surgical therapy. The buccal and lingual gingiva are evaluated separately to examine all aspects of gingival enlargement, including buccolingual and apicocoronal dimensions, probing depth, texture, color, density, shape, contour, and retractability of the gingival tissues.50 For these reasons, the Ingles index seems to be the most clinically appropriate and is the most currently employed system used to classify DIGE.
Disease Management
Treatment Sequencing
DIGE needs to be treated to reduce inflammation, decrease long-term effects of the disease, and eliminate patient discomfort. A proper diagnosis is necessary to devise an appropriate, personalized treatment plan, starting with a thorough medical and dental history evaluation, as illustrated in Figure 2. Information regarding social and family histories is important to determine the extent of risk factors. A review of drug history helps to identify the etiology of DIGE. After all this information has been documented, a full-mouth periodontal charting must be completed to determine the status of the periodontium. This should be done in conjunction with a diagnostic radiographic examination, including bitewing and periapical imaging.
Once a differential diagnosis has been established, the dental professional may choose to perform a biopsy and acquire a CBC to develop a working diagnosis. Once DIGE has been diagnosed and classified, treatment can commence. DIGE can be treated nonsurgically or surgically; however, nonsurgical therapy should be implemented first to minimize the invasiveness of treatment.54,55 Typically, nonsurgical management may suffice for grade 1 and grade 2 DIGE. For grade 3 and grade 4 DIGE, if this approach is deemed ineffective after a period of up to 12 months, treatment may then be escalated to surgical intervention.50,54,56 Surgical therapies should always be selected based on the most up-to-date clinical practice guidelines, along with individual functional and esthetic requirements of the patient (Table 3).54,55 Ultimately, dental professionals must use their best judgment while implementing the guidelines described above on a case-by-case basis.
Nonsurgical Therapy
The main goal of nonsurgical treatment is to reduce the inflammatory component of the disease in a non-invasive manner to avoid further complications.54 This may be achieved with adequate biofilm control. Routine periodontal recall examinations with SRP may suffice to eliminate inflammation by reducing the bacterial load. Periodontal re-evaluations should be performed to assess the effectiveness of SRP and guide future treatment decisions. Additionally, patient education with regular oral hygiene instructions is important for maintenance of good homecare and plaque control. Nonsteroidal anti-inflammatory drugs (NSAIDs) may be used as an adjunct with proper homecare to decrease collagen production and mediate the effects of gingival inflammation.4 Nevertheless, NSAIDs should be used sparingly and only when necessary.
The use of antibiotic treatment is not indicated in DIGE, because there is no evidence of its efficacy.57 However, short-term use of antibacterial rinses, such as chlorhexidine, in conjunction with good homecare techniques has been shown to be effective at maintaining plaque control for patients with DIGE.57,58 Furthermore, topical nystatin may be used in the treatment of papillary lesions on enlarged gingiva.4
In addition to plaque control, modification to the pharmacological regimen may be considered for patients with DIGE. It is important to note, however, that discontinuation of a drug in question can potentially do more harm than good. Therefore, drug substitutions should only be done in consultation with the patient and their family physician.59 Dentists should seek further information regarding the pharmacotherapeutic regimens for patients who present with hypertension, angina, epilepsy, or recent organ transplant. For patients on antihypertensive therapies with CCBs, nifedipine may be substituted for isradipine, which is a dihydropyrimidine derivative that is not known to be associated with DIGE.4 Alternatively, it is suggested to venture to another class of antihypertensive drug that is not known to cause gingival enlargement.4
Unlike the broad spectrum of antihypertensives, there are relatively fewer alternatives in immunosuppressive therapies. Among the few, tacrolimus has been known to spontaneously resolve DIGE when substituted for cyclosporine. For kidney transplant patients in particular, mycophenolic acid and azathioprine may be considered.4 These agents have demonstrated a protective effect against gingival enlargement, which is primarily attributed to their anti-proliferative and anti-inflammatory properties.4 Azithromycin may be used in combination with cyclosporine, as it has been shown to reduce DIGE and BOP.60 For patients taking anticonvulsant medications, phenytoin can be substituted with carbamazepine or valproic acid, both of which are less contributory to DIGE. Newer generations of anticonvulsants, such as lamotrigine, gabapentin, sulthiame, and topiramate have also proven to be effective in the reduction of DIGE compared to phenytoin.4
Typically, clinical improvements and regression of gingival enlargement can be seen as early as 4 weeks following drug substitution, in conjunction with good homecare59; however, it is important to allow 6 to 12 months for these changes to occur before making a decision to proceed to surgical treatment.4,54 Thus, by eliminating these modifiable risk factors, nonsurgical therapy aims to hinder the progression of DIGE and re-establish clinical gingival health. In cases of grade 3 and grade 4 DIGE, however, nonsurgical therapies may be insufficient to control the disease.
Surgical Therapy
Surgical therapies may be implemented in advanced cases of DIGE to reduce the burden of gingival enlargement on oral homecare and re-establish a healthy, maintainable periodontium. Typically, this may be achieved with open-flap debridement; in the case of severe DIGE, conventional/laser gingivectomy may be considered.4,54,56,57
Surgical therapies may be indicated in cases where drug substitution has failed or optimal plaque control cannot be achieved with conventional nonsurgical debridement techniques. In such cases, open-flap debridement may be performed to allow removal of persisting subgingival biofilm deposits. Resective surgical techniques may also be implemented to treat patients with DIGE. These procedures are particularly indicated when the presence of excess gingiva persists and interferes with function, esthetics, oral hygiene, and speech.54,57 In cases of marked overgrowth (ie, grade 3), flap surgery may be performed by making an internal bevel incision, raising a full-thickness envelope flap, and removing excessive underlying CT. This internal bevel technique is especially useful for patients with a reduced periodontium.57 For cases of severe overgrowth (ie, grade 4), an external bevel gingivectomy and soft-tissue recontouring may be more appropriate.57
Gingivectomy may be achieved by either conventional or laser techniques. Laser gingivectomy is an emerging technique used to treat DIGE.57 In fact, studies have shown the recurrence rate for carbon dioxide (CO2) laser gingivectomy was lower than that for conventional surgical methods.56,57 Furthermore, laser gingivectomy has the advantage of achieving faster hemostasis than conventional methods, which may enhance the visibility of the operative field and promote healing.57,61
Maintenance Therapy
Regardless of whether nonsurgical or surgical treatment is employed, maintenance therapies for DIGE are critical. During routine recall appointments, a rigorous cycle of SRP, patient education, and oral hygiene instruction must be implemented to maintain plaque control and prevent disease recurrence.57,62 Chlorhexidine gluconate can also be used as an antimicrobial adjunct to prevent recurrence.4,54 Surgical results may be maintained for 12 months, but recurrence can appear as early as 3 to 6 months after treatment.4,7,54
A longitudinal study by Fardal and Lygre showed that up to 47% of patients experienced a recurrence of DIGE at follow-up appointments.63 Of these patients, those who used CCBs had an increased rate of tooth loss compared to those who did not. In addition, those who substituted CCBs for an alternative drug demonstrated long-term improvements.57,63 In a prospective study to evaluate treatment outcomes, Ilgenli and colleagues found that the recurrence rate of DIGE was 34% during the first 18 months after periodontal surgery, regardless of drug use.56,57,64 These findings show that the recurrence of gingival enlargement can occur in 30% to 40% of patients in a short-term follow-up of less than 2 years.57 The incidence of recurrence may also be influenced by determining factors such as attendance to recall examination, age, and gingival inflammation levels following surgical therapy for severe DIGE.64 Ultimately, these longitudinal studies have identified the importance of maintenance therapies in relation to preventing the recurrence of DIGE.
Conclusion
DIGE is a multifactorial gingival condition associated with the use of CCBs, immunosuppressants, and anticonvulsants. Cannabis has been proposed as a potential inducing drug; however, high-quality evidence does not currently exist to support this. The major risk factors for DIGE include age, sex, genetics, drug and periodontal variables, and concomitant medications. Dental biofilm is not considered a risk factor for DIGE; instead, it is a fundamental cofactor in its etiology.
There are several localized and generalized gingival diseases and systemic conditions that manifest with a similar clinical appearance to DIGE. Although these diseases may coexist, it is important to distinguish DIGE from its clinical counterparts, as treatment strategies may contrast significantly. Once an accurate diagnosis of DIGE has been reached, the condition must be classified to evaluate its severity. Several indices exist for the classification of gingival overgrowth. It is up to the clinician to choose which clinical index to use to classify DIGE, although certain clinical indices are more relevant than others. Nonetheless, additional research is required to fine-tune these indices and correlate them with standardized treatment strategies for DIGE at different levels of severity.
First and foremost, the factors involved in the etiopathogenesis of DIGE should be addressed to guide appropriate treatments and reduce the risk of recurrence. Nonsurgical, surgical, and maintenance therapies are used to manage patients with DIGE. Priority should be given to nonsurgical therapy, which must be re-evaluated thoroughly before proceeding to surgical treatment. Surgical treatment of DIGE is supported for marked and severe overgrowths; however, clinicians should use their best judgment while planning treatment, always considering the functional and esthetic requirements of their patients. Ultimately, the goal of DIGE treatment is to re-establish a healthy, maintainable periodontium and interrupt the positive feedback cycle that results in persistent gingival enlargement. In doing so, patients can regain functionality, esthetics, and their quality of life.
About the Authors
Fady Barsoum
Fourth-Year Student, Doctor of Dental Surgery (DDS) Program at the Faculty of Dentistry, University of Toronto, Toronto, Ontario, Canada
Braedan R.J. Prete, BSc (Hons)
Fourth-Year Student, Doctor of Dental Surgery (DDS) Program at the Faculty of Dentistry, University of Toronto, Toronto, Ontario, Canada
Aviv Ouanounou, BSc, MSc, DDS
Associate Professor, Department of Clinical Sciences (Pharmacology and Preventive Dentistry), Faculty of Dentistry, University of Toronto, Toronto, Ontario, Canada; Fellow, International College of Dentists; Fellow, American College of Dentists; Fellow, International Congress of Oral Implantologists
Queries to the author regarding this course may be submitted to authorqueries@aegiscomm.com.
References
1. Murakami S, Mealey BL, Mariotti A, Chapple ILC. Dental plaque-induced gingival conditions. J Periodontol. 2018;89(suppl 1):S17-S27.
2. Hughes FJ, Bartold PM. Periodontal complications of prescription and recreational drugs. Periodontol 2000. 2018;78(1):47-58.
3. Dongari-Bagtzoglou A. Drug-associated gingival enlargment. J Periodontol. 2004;75(10):1424-1431.
4. Bharti V, Bansal C. Drug-induced gingival overgrowth: the nemesis of gingiva unravelled. J Indian Soc Periodontol. 2013;17(2):182-187.
5. Tungare S, Paranjpe AG. Drug-induced gingival overgrowth. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2022. PMID: 30860753.
6. Seymour RA, Thomason JM, Ellis JS. The pathogenesis of drug-induced gingival overgrowth. J Clin Periodontol. 1996;23(3 Pt 1):165-175.
7. Livada R, Shiloah J. Calcium channel blocker-induced gingival enlargement. J Hum Hypertens. 2014;28(1):10-14.
8. Barclay S, Thomason JM, Idle JR, Seymour RA. The incidence and severity of nifedipine-induced gingival overgrowth. J Clin Periodontol. 1992;19(5):311-314.
9. Duncan MR, Berman B. Stimulation of collagen and glycosaminoglycan production in cultured human adult dermal fibroblasts by recombinant human interleukin 6. J Invest Dermatol. 1991;97(4):686-692.
10. Pernu HE, Knuuttila ML, Huttunen KR, Tiilikainen AS. Drug-induced gingival overgrowth and class II major histocompatibility antigens. Transplantation. 1994;57(12):1811-1813.
11. Seymour RA, Ellis JS, Thomason JM. Risk factors for drug-induced gingival overgrowth. J Clin Periodontol. 2000;27(4):217-223.
12. Guncu GN, Caglayan F, Dincel A, et al. Clinical and pharmacological variables as a risk factor for nifedipine-induced gingival overgrowth. Aust Dent J. 2007;52(4):295-299.
13. Sharma A, Joshi S, Rana SRL, et al. Amlodipine induced gingival overgrowth in patients at a tertiary level hospital of Nepal. J Nepal Soc Periodontol Oral Implantol. 2018;2(1):2-5.
14. Jorgensen MG. Prevalence of amlodipine-related gingival hyperplasia. J Periodontol. 1997;68(7):676-678.
15. Kothari L, Bajaj P, Jaiswal P, Agrawal D. Prevalence of amlodipine induced gingival enlargement - a hospital based study. J Evolution Med Dent Sci. 2020;9(34):2432-2435.
16. Agrawal AA. Gingival enlargements: differential diagnosis and review of literature. World J Clin Cases. 2015;3(9):779-788.
17. Gaur S, Agnihotri R. Is dental plaque the only etiological factor in amlodipine induced gingival overgrowth? A systematic review of evidence. J Clin Exp Dent. 2018;10(6):e610-e619.
18. Rees TD, Levine RA. Systematic drugs as a risk factor for periodontal disease initiation and progression. Compendium. 1995;16(1):20-26.
19. Lederman D, Lumerman H, Reuben S, Freedman PD. Gingival hyperplasia associated with nifedipine therapy: report of a case. Oral Surg Oral Med Oral Pathol. 1984;57(6):620-622.
20. Miller CS, Damm DD. Incidence of verapamil-induced gingival hyperplasia in a dental population. J Periodontol. 1992;63(5):453-456.
21. Kataoka M, Kido J, Shinohara Y, Nagata T. Drug-induced gingival overgrowth - a review. Biol Pharm Bull. 2005;28(10):1817-1821.
22. Greenberg KV, Armitage GC, Shiboski CH. Gingival enlargement among renal transplant recipients in the era of new-generation immunosuppressants. J Periodontol. 2008;79(3):453-460.
23. Ponnaiyan D, Jegadeesan V. Cyclosporine A: novel concepts in its role in drug-induced gingival overgrowth. Dent Res J (Isfahan). 2015;12(6):499-506.
24. Barbarino JM, Staatz CE, Venkataramanan R, et al. PharmGKB summary: cyclosporine and tacrolimus pathways. Pharmacogenet Genomics. 2013;23(10):563-585.
25. Hernandez G, Arriba L, Lucas M, de Andres A. Reduction of severe gingival overgrowth in a kidney transplant patient by replacing cyclosporin A with tacrolimus. J Periodontol. 2000;71(10):1630-1636.
26. James JA, Boomer S, Maxwell AP, et al. Reduction in gingival overgrowth associated with conversion from cyclosporin A to tacrolimus. J Clin Periodontol. 2000;27(2):144-148.
27. Rick J, Thompson AM, Hsiao JL, et al. Immunosuppressants, immunomodulators and COVID-19 vaccines: anticipating patient concerns. J Dermatolog Treat. 2021;1-4. doi: 10.1080/09546634.2021.1880543.
28. Chiricozzi A, Gisondi P, Bellinato F, Girolomoni G. Immune response to vaccination in patients with psoriasis treated with systemic therapies. Vaccines (Basel). 2020;8(4):769.
29. Palestine AG, Roberge F, Charous BL, et al. The effect of cyclosporine on immunization with tetanus and keyhole limpet hemocyanin (KLH) in humans. J Clin Immunol. 1985;5(2):115-121.
30. Versluis DJ, Beyer WE, Masurel N, et al. Impairment of the immune response to influenza vaccination in renal transplant recipients by cyclosporine, but not azathioprine. Transplantation. 1986;42(4):376-379.
31. Devaux CA, Melenotte C, Piercecchi-Marti MD, et al. Cyclosporin A: a repurposable drug in the treatment of COVID-19? Front Med (Lausanne). 2021;8;663708.
32. Birse F, Derry S, Moore RA. Phenytoin for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2012;2012(5):CD009485.
33. Chacko LN, Abraham S. Phenytoin-induced gingival enlargement. BMJ Case Rep. 2014;2014:bcr2014204670.
34. Mohan RP, Rastogi K, Bhushan R, Verma S. Phenytoin-induced gingival enlargement: a dental awakening for patients with epilepsy. BMJ Case Rep. 2013;2013:bcr2013008679.
35. Guncu GN, Caglayan F, Dincel A, et al. Plasma and gingival crevicular fluid phenytoin concentrations as risk factors for gingival overgrowth. J Periodontol. 2006;77(12):2005-2010.
36. Joshipura V. Sodium valproate induced gingival enlargement with pre-existing chronic periodontitis. J Indian Soc Periodontol. 2012;16(2):278-281.
37. Dhalkari CD, Ganatra PV. Sodium valproate induced gingival enlargement in 22 months old child. J Indian Soc Periodontol. 2014;18(5):644-647.
38. Rawal SY, Dabbous MK, Tipton DA. Effect of cannabidiol on human gingival fibroblast extracellular matrix metabolism: MMP production and activity, and production of fibronectin and transforming growth factor B. J Periodontal Res. 2012;47(3):320-329.
39. Rawal SY, Dimitris NT, Tipton DA. Periodontal and oral manifestations of marijuana use. J Tenn Dent Assoc. 2012;92(2):26-31.
40. Dhakal R, Chaudary SK, Singh R, et al. Marijuana induced gingival enlargement. J Nepal Soc Perio Oral Implantol. 2019;3(5):25-28.
41. Newman MG, Takei H, Klokkevold PR, Carranza FA. Carranza's Clinical Periodontology. St. Louis, MO: Saunders Elsevier; 2006:375-376.
42. Chee WW, Jansen CE. Phenytoin hyperplasia occurring in relation to titanium implants: a clinical report. Int J Oral Maxillofac Implant. 1994;9(1):107-109.
43. Silverstein LH, Koch JP, Lefkove MD, et al. Nifedipine-induced gingival enlargement around dental implants: a clinical report. J Oral Implantol. 1995;21(2):116-120.
44. Quach H, Ray-Chaudhuri A. Calcium channel blocker induced gingival enlargement following implant placement in a fibula free flap reconstruction of the mandible: a case report. Int J Implant Dent. 2020;6(1):47.
45. Caton JG, Armitage G, Berglundh T, et al. A new classification scheme for periodontal and peri-implant diseases and conditions - introduction and key changes from the 1999 classification. J Periodontol. 2018;89(suppl 1):S1-S8.
46. Bharanidharan R, Dineshkumar T, Raghavendhar K, Kumar AR. Squamous cell carcinoma of the gingiva: a diagnostic enigma. J Oral Maxillofac Pathol. 2015;19(2):267.
47. Cabral LA, de Carvalho LF, Salgado JA, et al. Gingival squamous cell carcinoma: a case report. J Oral Maxillofac Res. 2010;1(3):e6.
48. Samudrala P, Chava VK, Chandana TS, Suresh R. Drug-induced gingival overgrowth: a critical insight into case reports from over two decades. J Indian Soc Periodontol. 2016;20(5):496-502.
49. Gossweiler AG, Martinez-Mier E. Vitamins and oral health. In: Zohoori FV, Duckworth RM, eds. The Impact of Nutrition and Diet on Oral Health. Basel, Switzerland: Karger; 2020:59-67.
50. Ingles E, Rossman JA, Caffesse RG. New clinical index for drug-induced gingival overgrowth. Quintessence Int. 1999;30(7):467-473.
51. Dubey S, Gattani D, Deotale S, Quazi M. A contemporary review on indices for gingival enlargement. J Adv Med Dent Sci Res. 2016;4(5):62-67.
52. Miranda J, Brunet L, Roset P, et al. Reliability of two measurement indices for gingival enlargement. J Periodontal Res. 2012;47(6):776-782.
53. Seymour RA, Smith DG, Turnbull DN. The effects of phenytoin and sodium valproate on the periodontal health of adult epileptic patients. J Clin Periodontol. 1985;12(6):413-419.
54. Camargo PM, Melnick PR, Pirih FQ, et al. Treatment of drug-induced gingival enlargement: aesthetic and functional considerations. Periodontol 2000. 2001;27:131-138.
55. Aimetti M, Romano F, Debernardi C. Effectiveness of periodontal therapy on the severity of cyclosporin A-induced gingival overgrowth. J Clin Periodontol. 2005;32(8):846-850.
56. Mavrogiannis M, Ellis JS, Seymour RA, Thomason JM. The efficacy of three different surgical techniques in the management of drug-induced gingival overgrowth. J Clin Periodontol. 2006;33(9):677-682.
57. Zoheir N, Hughes FJ. The management of drug-influenced gingival enlargement. Prim Dent J. 2020;8(4):34-39.
58. O'Neil TC, Figures KH. The effects of chlorhexidine and mechnical mathods of plaque control on the recurrence of gingival hyperplasia in young patients taking phenytoin. Br Dent J. 1982;152(4):130-133.
59. Harel-Raviv M, Eckler M, Lalani K, et al. Nifedipine-induced gingival hyperplasia. A comprehensive review and analysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79(6):715-722.
60. Teshome A, Girma B, Aniley Z. The efficacy of azithromycin on cyclosporine-induced gingival enlargement: systematic review and meta-analysis. J Oral Biol Craniofacial Res. 2020;10(2):214-219.
61. Goharkhay K, Moritz A, Wilder-Smith P, et al. Effects on oral soft tissue produced by a diode laser in vitro. Lasers Surg Med. 1999;25(5):401-406.
62. Moffitt M, Bencivenni D, Cohen R. Treatment modalities for drug-induced gingival enlargement. J Dent Hyg. 2012;86(4):272-277.
63. Fardal O, Lygre H. Management of periodontal disease in patients using calcium channel blockers - gingival overgrowth, prescribed medications, treatment responses and added treatment costs. J Clin Periodontol. 2015;42(7):640-646.
64. Ilgenli T, Atilla G, Baylas H. Effectiveness of periodontal therapy in patients with drug-induced gingival overgrowth. Long-term results. J Periodontol. 1999;70(9):967-972.