You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
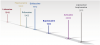
Safe and effective local anesthesia is one of the most important advancements in dentistry, paving the way for improved treatment experiences and, ultimately, better patient care.1,2 Comprehensive intraoperative pain control utilizing local anesthesia allows complex dental treatments to be performed successfully. Without it, most patients could not tolerate routine treatment, and this would necessitate general anesthesia in a hospital setting for invasive care.2,3 The modern era of local anesthesia has been characterized by an armamentarium of excellent medication options since 1948, when lidocaine first received approval by the US Food and Drug Administration (FDA) for use in the United States (Figure 1).3,4 These medications now facilitate ease of use and safety, yet the search continues for new solutions and techniques to improve clinical efficacy.4
Despite clinicians having several effective local anesthetics at their disposal, the administration of local anesthesia is usually anxiety-provoking and often unpleasant for the dental patient. Subjective reports of injection pain from tissue penetration of the needle or stinging and burning from the solution are common. Techniques to help mitigate injection pain and improve the patient experience may include changes to the armamentarium, alkalinizing or warming the anesthetic solution, the use of vibration or application of pressure for distraction, and slow injection techniques. However, a study by Meit and colleagues indicated that while many dental practitioners are aware of these techniques, they are not universally adopted.5 Given that invasive dentistry cannot proceed without effective local anesthesia delivery, and that patient assessment of dental providers is often associated with injection comfort, it is not hyperbole to acknowledge the importance of painless and profound local anesthesia.6 This article will review four strategies clinicians may consider to take their local anesthesia to the next level (Table 1).
Effects of Warming or Cooling Local Anesthetic Solutions
Some researchers recommend warming local anesthetic solutions, while others believe it offers no benefits, and the mechanism is still unclear as to why warming may decrease injection pain.7 Warming of local anesthetic solutions prior to injection was first described in a letter by Boggia in 1967; suggested mechanisms of action include nociceptor stimulation, based on the belief that cold is more painful than warm, increased solubility of the solution, and changes of the pKa to create a more basic form of the anesthetic to decrease latency.8-10 Practitioners can use low-tech methods for warming a dental local anesthetic cartridge before injection, such as holding it in the hand for a few minutes to warm it via body heat or placing it in a cup of warm water. Alternatively, cartridge warming devices may be used to achieve a recommended temperature for the warmed cartridge contents of 37°C to 43°C.11,12
In an early double-blind crossover study by Davidson and Boom involving 25 subjects who received 1% lidocaine subcutaneously, 89% responded that the room temperature formulation (20°C) was more painful than the warmed anesthetic formulation at 37°C to 43°C.7 A study by Lundbom et al involved 36 healthy volunteers who received three injections of 4.5 mL 1% lidocaine subcutaneously into the abdomen: one refrigerated at 8°C, one at room temperature at 21°C, and one warmed to 37°C.12 When subjects rated their pain from most painful to least painful the warmed local anesthetic was found to be the least painful, the cooled formulation was intermediate, and the most painful was room temperature. It is interesting that refrigerated lidocaine was involved in the study, because in most cases injections at room temperature versus warmed are compared. The authors concluded that warming of lidocaine to body temperature should always be considered before injection and that storing lidocaine in the refrigerator does not increase injection pain.
Although research on cooling local anesthetics is scarce and less compelling, a study by Dabarakis et al examined the effect of temperature on the onset and duration of pulpal anesthesia using 3% mepivacaine.13 Following injection of mepivacaine at room temperature (20°C) and cooled (4°C), there was no statistical difference in the onset of anesthesia among the subjects but the cooled anesthetic showed a statistically significant increase in duration (29% increase). Measurement of injection pain was not an outcome of the study, however the authors stated that the majority of the subjects mentioned experiencing more pain during the cold injection.
A meta-analysis by Hogan and colleagues found a reduction in pain from warmed anesthetics when they were injected subcutaneously or intradermally. Their conclusion that, "no significant benefit was observed for intraoral injections," may be incomplete, however, because most of the included studies involved injections outside the mouth, with only one study involving intraoral injections.14 In a split-mouth randomized clinical trial by Aravena et al in which 72 patients received either a warmed or room temperature injection of 0.9 mL of 2% lidocaine with 1:100,000 epinephrine in the buccal vestibule of the maxillary lateral incisal area, results showed that warmed local anesthetic resulted in significantly reduced pain compared to room temperature injections.11 More recently, Gumus and Aydinbelge conducted a double-blind, split-mouth clinical study comparing the pain perception of room temperature (21°C) versus warmed (37°C) articaine in children aged 5 to 8 years.15 One hundred subjects received a maxillary buccal infiltration, and the results showed a statistically significant reduction in pain perception and heart rate when the warmed local anesthetic was used.
Vibration and Distraction
In 1965, Melzack and Wall described the gate control theory, explaining a pain-modulating system in which a neural "pain gate" present in the spinal cord can open and close, thereby modulating the perception of pain.16 Some nerve fibers in the body transmit pain (eg, type A delta and type C dorsal root fibers), while others can transmit touch or pressure (eg, type A beta fibers). In situations where both painful and pressure stimuli are felt, the dual transmissions of sensations race to the brain to be interpreted, each by different nerve tracks. According to the gate control theory if a non-painful stimulus reaches the brain first neural gates will close and the non-painful stimulus will override the painful stimulus thereby decreasing the perception of pain. The smaller, unmyelinated type A delta and type C nerve fibers that transmit pain sensations are susceptible to nerve block via local anesthetics.17 Larger, myelinated type A beta fibers transmit touch, temperature, and pressure sensations, and these impulses are transmitted faster than unmyelinated nerve fibers. Type A beta fibers can be stimulated by wiggling the patient's cheek during local anesthetic administration or through the use of a vibrating device. Literature on the use of vibrating devices to improve patient comfort during local anesthesia administration is generally positive but equivocal.18-22
Three examples of vibrating devices are: transcutaneous electronic nerve stimulation (TENS) units, which pass a high-frequency, low-voltage, electric current between two electrodes to activate the type A beta fibers, sending signals to the brain that block or scramble normal pain signals; a vibrating device that snaps on to the barrel of an existing metal syringe; and a cordless, rechargeable handheld wand featuring tips that vibrate.
A study by Ching et al compared pain-rating scale measurements in a split-mouth study in 36 adolescent patients aged 10 to 17 years. Each patient received two infiltration injections, one of which involved the use of a vibrating device (the order of injection was "no vibration" then "vibration" to rule out an ordered effect), and immediately after the injections the amount of discomfort was rated from 0 to 10 using the Wong-Baker FACES pain rating scale.18 The median difference between pain felt by the two groups was two, with 17 of the patients reporting zero pain on injection with the vibrating device, compared to only three by the control group. The authors concluded that most subjects in the vibration group (83%) reported significantly less pain than in the control group. This study supports the earlier work of Nanitsos et al where it was concluded that, "applied vibration decreases pain associated with a local anesthetic injection"; however, in the Nanitsos study the vibration stimulus was applied extraorally by the patient during the time of the injection.21
In a study by Nasehi et al, researchers compared the anticipated and actual pain of seven different dental injections. Pain value was recorded using the 0-10 visual analog score (VAS) following bilateral injections, one with a vibrating device and one without.19 In one example, when subjects, who were given a brief explanation about the study, were asked to rate the anticipated pain from the inferior alveolar nerve block (IANB) injection without vibration the mean was 5.37 and the actual was 5.26. With vibration, the anticipated pain from the IANB injection was 2.85, and actual was 1.73. Interestingly, patients not only thought the injection would hurt less when vibration was used, but the actual pain was even less than anticipated. The researchers concluded that VAS scores were significantly lower when the vibration device was used during local anesthetic injection.
A study by Shaefer used the symptom severity index (SSI), including the VAS, to not only evaluate pain but also inquire about the experience of the injection with the practitioner using a vibrating device.20 In 60 subjects receiving an IANB injection there was a significant difference in both SSI scores (intensity of discomfort, unpleasantness, and ease of enduring the injection) and VAS. The authors concluded the vibrating device, "reduced pain from dental anesthesia when used with injections that are routinely difficult for patients to tolerate," such as the IANB injection.
Liposomal Encapsulation
Liposomal bupivacaine has garnered recent interest as a possible therapy to help reduce reliance on opioids for postoperative pain management.23 Known commercially as bupivacaine liposome injectable suspension, this formulation is founded on a proprietary extended-release drug delivery technology.24 Microscopic spherical particles composed of internal aqueous chambers contain the active drug, and lipid membranes separate the individual chambers from each other.25 As the lipid membranes dissolve and reorganize, they release bupivacaine over an extended period. Release rates are determined by the relative amounts of lipids and proprietary manufacturing technology.
The onset of action for liposomal bupivacaine is similar to that of conventional bupivacaine, with profound local anesthesia generally occurring within 5 minutes. While the manufacturer's data from a single-blind study showed that the extended-release drug delivery technology increases the overall duration of effect to up to 96 hours,23,26 this study26 is controversial and other investigators suggest that the clinical response, and not just area-under-the-curve data, shows a much shorter duration of action.27In addition, Davidson et al found that the time to maximum concentration in blood was 262 ± 149 minutes for bupivacaine liposome injectable suspension compared to 37 ± 16 minutes for plain bupivacaine.28
While the clinical trials that brought the bupivacaine liposome injectable suspension drug to market never targeted it for use in dental procedures, there is a growing number of oral healthcare professionals who have used this medication to provide extended local anesthesia in the hope of mitigating postoperative opioid prescribing. In fact, a recent retrospective cross-sectional study by Lieblich et al of the effect of liposomal bupivacaine on postoperative opioid prescribing after third molar extraction found that the patients receiving the bupivacaine liposome injectable suspension were prescribed significantly fewer opioids than patients who did not receive the drug, with a lower refill rate.29 (Patients were instructed to initiate postsurgical analgesia at home with 600 mg of ibuprofen and to repeat this dose every 6 hours for at least 48 hours. Breakthrough pain was treated with acetaminophen 650 mg.) The Lieblich study had noteworthy limitations as it was retrospective in nature and procedures conducted without bupivacaine liposome injectable suspension were all performed in 2012, while the procedures conducted with bupivacaine liposome injectable suspension at the same center were performed 6 years later in 2018. Regardless, the authors concluded that the use of bupivacaine liposome injectable suspension may help to reduce opioid prescriptions for postsurgical analgesia. However, this strategy should not be considered routine or even standard of care given other potential safety issues associated with the drug (eg, persistent anesthesia, paresthesias, weakness, and muscle paralysis), as well as the high current average wholesale price for a single cartridge compared to that of conventional local anesthetics, such as 0.5% bupivacaine plus 1:200,000 epinephrine.30
Although liposomal bupivacaine may present a unique opportunity to prolong local anesthetic action after intraoral injection, more peer-reviewed studies are needed to establish its safety and efficacy compared to existing strategies.
Buffering
Buffering or alkalinization of dental local anesthetics to raise the pH of these acidic solutions is a well-documented technique that results in clinical benefits such as reduced onset time, decreased injection pain, and the need for less overall volume of local anesthesia.31-34 The pH range of commercially available local anesthetic solutions containing a vasoconstrictor such as epinephrine is between 3 and 5, and this low pH may contribute to injection-site pain and slow onset.35 To mitigate the adverse effects of these acidic local anesthetic solutions, the addition of 8.4% sodium bicarbonate to buffer or alkalinize these solutions closer to physiologic pH has been extensively studied in dentistry and medicine.34,36-41 Buffering or alkalinization of these solutions drives the stoichiometric relationship toward more uncharged local anesthetic molecules in situ. Because these molecules are lipid soluble, they readily cross lipid membranes, resulting in faster, more profound, and more effective local anesthesia clinically. The results of a recent systematic analysis showed that buffered local anesthetics are more effective than nonbuffered local anesthetics when used for mandibular or maxillary anesthesia in pulpally involved teeth, and that buffered local anesthetics have 2.29 times greater likelihood of achieving successful anesthesia.32
A second mechanism of action was first postulated by Condouris and Shakalis back in 1964.42 These investigators confirmed that buffering a local anesthetic with sodium bicarbonate generates carbon dioxide (CO2), which also has local anesthetic activity on its own, something that Necheles and Gerard first showed in 1930.43 CO2 easily traverses the nerve sheath and membrane into the axoplasm. Some of the CO2 combines with water to form carbonic acid within the axoplasm, lowering the axoplasm pH. When the uncharged local anesthetic crosses into the axoplasm, because of the lower pH it charges up and remains trapped in axoplasm (on the inside of sodium) for a longer period of time, possibly leading to enhanced local anesthesia. This is a distinct mechanism whereby just increasing the pH of the local anesthetic drives it into the axoplasm faster.
On the horizon, FDA approval is being sought for new buffered local anesthetics that offer the promise of overcoming the current barrier to adoption of buffered local anesthetics: admixture at chairside. If these products are supplied in a standard 1.7 mL dental cartridge and at a cost more comparable to current non-buffered drugs, they could represent the next generation and new standard for local anesthetics in dentistry. In addition, removal of sodium chloride from the formulation will significantly reduce the current hypertonicity of buffered mixtures (approximately 217 mOsm/L), which will further contribute to patient comfort. Perhaps most importantly, the possibility of local toxicity or sterility breaches due to current "chairside compounding" techniques will be completely eliminated. This is significant, as 8.4% sodium bicarbonate has an osmolality of 2,000 mOsm/L, and chairside compounding adds additional failure points in the sterility chain.44
Conclusion
With an increased number of available local anesthetic solutions, coupled with choices in techniques, delivery systems, and even the ability to alkalinize the solutions for improved onset and efficacy, oral healthcare providers have many tools to mitigate peri-operative dental pain. For providers who are still challenged with obtaining good, profound local anesthesia, this article has described four additional possibilities to consider to raise their administration of local anesthesia to the next level.
About the Authors
Mark Donaldson, BSP, ACPR, PharmD
Associate Principal, Vizient Pharmacy Advisory Solutions, Irving, Texas; Clinical Professor, School of Pharmacy, University of Montana, Missoula, Montana; Clinical Assistant Professor, School of Dentistry, Oregon Health & Sciences University, Portland, Oregon; Adjunct Professor, Faculty of Dentistry, University of British Columbia, Vancouver, British Columbia; Fellow, American Society of Health-System Pharmacists; Fellow, American College of Healthcare Executives®
Jason H. Goodchild, DMD
Vice President of Clinical Affairs, Premier Dental Products Co., Plymouth Meeting, Pennsylvania; Associate Clinical Professor, Department of Oral and Maxillofacial Surgery, Creighton University School of Dentistry, Omaha, Nebraska; Adjunct Assistant Professor, Division of Oral Diagnosis, Department of Diagnostic Sciences, Rutgers School of Dental Medicine, New Brunswick, New Jersey
Queries to the author regarding this course may be submitted to authorqueries@aegiscomm.com.
References
1. Gordh T. Lidocaine: the origin of a modern local anesthetic. 1949. Anesthesiology. 2010;113(6):1433-1437.
2. Malamed SF. Local anesthetics: dentistry's most important drugs, clinical update 2006. J Calif Dent Assoc. 2006;34(12):971-976.
3. Donaldson M, Goodchild JH. Lidocaine turns 70: the evolution of dental local anesthesia. Gen Dent. 2018;66(3):6-9.
4. Drugs@FDA: FDA-Approved Drugs. US Food & Drug Administration website. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=021381. Accessed November 2, 2021.
5. Meit SS, Yasek V, Shannon CK, et al. Techniques for reducing anesthetic injection pain: an interdisciplinary survey of knowledge and application. J Am Dent Assoc. 2004;135(9):1243-1250.
6. de St Georges J. How dentists are judged by patients. Dent Today. 2004;23(8):96,98-99.
7. Davidson JA, Boom SJ. Warming lignocaine to reduce pain associated with injection. BMJ. 1992;305(6854):617-618.
8. Boggia R. Heating local anaesthetic cartridges (letter). Br Dent J. 1967;122:287.
9. Martin S, Jones JS, Wynn BN. Does warming local anesthetic reduce the pain of subcutaneous injection? Am J Emerg Med. 1996;14(1):10-12.
10. Finsen V. Reduced pain when injecting lidocaine. Tidsskr Nor Laegeforen. 2017;137(9):629-630.
11. Aravena PC, Barrientos C, Troncoso C, et al. Effect of warming anesthetic on pain perception during dental injection: a split-mouth randomized clinical trial. Local Reg Anesth. 2018;11:9-13.
12. Lundbom JS, Tangen LF, Wago KJ, et al. The influence of lidocaine temperature on pain during subcutaneous injection. J Plastic Surg Hand Surg. 2017;51(2):118-121.
13. Dabarakis N, Tsirlis A, Parisis N, Tsoukalas D. The role of temperature in the action of mepivacaine. Anesth Prog. 2006;53(3):91-94.
14. Hogan ME, vanderVaart S, Perampaladas K, et al. Systematic review and meta-analysis of the effect of warming local anesthetics on injection pain. Ann Emerg Med. 2011;58(1):86-98.
15. Gumus H, Aydinbelge M. Evaluation of effect of warm local anesthetics on pain perception during dental injections in children: a split-mouth randomized clinical trial. Clin Oral Investig. 2020;24(7):2315-2319.
16. Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150(3699):971-979.
17. Drasner K. Local anesthetics. In: Katzung BG, Masters SB, Trevor AJ, eds. Basic and Clinical Pharmacology. 12th ed. New York, NY: McGraw-Hill Medical; 2012:455.
18. Ching D, Finkelman M, Loo CY. Effect of the DentalVibe injection system on pain during local anesthesia injections in adolescent patients. Pediatr Dent. 2014;36(1):51-55.
19. Nasehi A, Bhardwaj S, Kamath AT, et al. Clinical pain evaluation with intraoral vibration device during local anesthetic injections. J Clin Exp Dent. 2015;7(1)e23-e27.
20. Shaefer JR, Lee SJ, Anderson NK. A vibration device to control injection discomfort. Compend Contin Educ Dent. 2017;38(6):e5-e8.
21. Nanitsos E, Vartuli R, Forte A, et al. The effect of vibration on pain during local anaesthesia injections. Aust Dent J. 2009;54(2):94-100.
22. Saijo M, Ito E, Ichinohe T, Kaneko Y. Lack of pain reduction by a vibrating local anesthetic attachment: a pilot study. Anesth Prog. 2005;52(2):62-64.
23. Exparel® [package insert]. Parsippany, NJ: Pacira Pharmaceuticals. Updated April 2018. https://www.exparel.com/sites/default/files/EXPAREL_PI_Nerve_Block_Final_4-6-2018.pdf. Accessed November 2, 2021.
24. Lambert WJ, Los K. DepoFoam multivesicular liposomes for the sustained release of macromolecules. In: Rathbone MJ, Hadgraft J, Roberts MS, Lane ME, eds. Modified-Release Drug Delivery Technology. Vol 2. 2nd ed. New York, NY: Informa Healthcare; 2008.
25. How DepoFoam works. Pacira Pharmaceuticals website. 2018. https://www.pacira.com/products/depofoam. Accessed November 2, 2021.
26. Apseloff G, Onel E, Patou G. Time to onset of analgesia following local infiltration of liposome bupivacaine in healthy volunteers: a randomized, single-blind, sequential cohort, crossover study. Int J Clin Pharmacol Ther. 2013;51(5):367-373.
27. Hadley RM, Dine AP, Where is the evidence? A critical review of bias in the reporting of clinical data for Exparel: a liposomal bupivacaine formulation. J Clinic Res Bioeth. 2014;5(4):1000189.
28. Davidson EM, Barenholz Y, Cohen R, et al. High-dose bupivacaine remotely loaded into multivesicular liposomes demonstrates slow drug release without systemic toxic plasma concentrations after subcutaneous administration in humans. Anesth Analg. 2010;110(4):1018-1023.
29. Lieblich SE, Misiek D, Olczak J, et al. A retrospective cross-sectional study of the effect of liposomal bupivacaine on postoperative opioid prescribing after third molar extraction. J Oral Maxillofac Surg. 2021;79(7):1401-1408.e1.
30. Goodchild JH, Donaldson M. Does liposomal bupivacaine fulfill an unmet need in dentistry? Gen Dent. 2018;66(5):14-16.
31. Goodchild JH, Donaldson M. Novel direct injection chairside buffering technique for local anesthetic use in dentistry. Compend Contin Educ Dent. 2019;40(7):e1-e10.
32. Kattan S, Lee SM, Hersh EV, Karabucak B. Do buffered local anesthetics provide more successful anesthesia than nonbuffered solutions in patients with pulpally involved teeth requiring dental therapy?: A systematic review. J Am Dent Assoc. 2019;150(3):165-177.
33. Goodchild JH, Donaldson M. Comparing the pH change of local anesthetic solutions using two chairside buffering techniques. Compend Contin Educ Dent. 2016;37(5):e6-e12.
34. Cepeda MS, Tzortzopoulou A, Thackrey M, et al. Adjusting the pH of lidocaine for reducing pain on injection [update in Cochrane Database Syst Rev. 2015;5:CD006581]. Cochrane Database Syst Rev. 2010;(12):CD006581.
35. Whitcomb M, Drum M, Reader A, et al. A prospective, randomized, double-blind study of the anesthetic efficacy of sodium bicarbonate buffered 2% lidocaine with 1:100,000 epinephrine in inferior alveolar nerve blocks. Anesth Prog. 2010;57(2):59-66.
36. McKay W, Morris R, Mushlin P. Sodium bicarbonate attenuates pain on skin infiltration with lidocaine, with or without epinephrine. Anesth Analg. 1987;66(6):572-574.
37. Stewart JH, Chinn SE, Cole GW, Klein JA. Neutralized lidocaine with epinephrine for local anesthesia-II. J Dermatol Surg Oncol. 1990;16(9):842-845.
38. Capogna G, Celleno D, Laudano D, Giunta F. Alkalinization of local anesthetics. Which block, which local anesthetic? Reg Anesth. 1995;20(5):369-377.
39. Curatolo M, Petersen-Felix S, Arendt-Nielsen L, et al. Adding sodium bicarbonate to lidocaine enhances the depth of epidural blockade. Anesth Analg. 1998;86(2):341-347.
40. Burns CA, Ferris G, Feng C, et al. Decreasing the pain of local anesthesia: a prospective, double-blind comparison of buffered, premixed 1% lidocaine with epinephrine versus 1% lidocaine freshly mixed with epinephrine. J Am Acad Dermatol. 2006;54(1):128-131.
41. Al Shahwan MA. Prospective comparison between buffered 1% lidocaine-epinephrine and skin cooling in reducing the pain of local anesthetic infiltration. Dermatol Surg. 2012;38(10):1654-1659.
42. Condouris GA, Shakalis A. Potentiation of the nerve-depressant effect of local anaesthetics by carbon dioxide. Nature. 1964;204:57-58.
43. Necheles H, Gerard RW: The effect of carbon dioxide on nerve. Am J Physiol. 1930;93(1):318-336.
44. Senewiratne NL, Woodall A, Can AS. Sodium bicarbonate. [Updated Nov 29, 2020]. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2021. https://www.ncbi.nlm.nih.gov/books/NBK559139/. Accessed November 2, 2021.