You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
COVID-19 is an acute respiratory disease caused by infection with the coronavirus SARS-CoV-2. Since initial reports of its emergence in December 2019, COVID-19 has spread worldwide, with over 143 million cases and 3 million deaths globally,1 and more than 31 million cases and 565,000 deaths in the United States alone, as of this writing.2 COVID-19 is thought to be spread primarily by infectious virus particles carried in aerosol droplets, a mechanism that is amplified in indoor settings with poor ventilation, particularly where groups of people have prolonged exposure to infected individuals. After initial infection, the virus has an approximately 5- to 6-day incubation period, and the response to infection varies widely, ranging from asymptomatic infections (up to 20% of cases) to severe infections resulting in prolonged hospitalization or death.3
The emergence of this deadly and widespread disease has prompted an extraordinary response on the part of the scientific community, with initial sequencing of the virus, characterization of the human immune response, and development of testing, therapeutics, and vaccines occurring on an unprecedentedly short timescale. No effort has exemplified this more than the worldwide vaccine development. As of mid-March 2021, roughly a year after the first shutdowns occurred in the United States, there were 21 vaccines in phase 3 trials and 12 vaccines that had been granted either emergency use or full approval.
This brief review will discuss the leading vaccines. It will first provide a description of SARS-CoV-2 and the main features of the human immune response to it, and then offer a breakdown of the leading vaccines (phase 3 and/or authorized for use) by mechanism of action, tabulating key measurements of immune response and phase 3 efficacy data. This will be followed by a short review of SARS-CoV-2 variants and their impact on vaccine efficacy, and, finally, a brief discussion of COVID-19 transmission risk after vaccination.
SARS-CoV-2: Structure and Immune Response
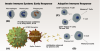
SARS-CoV-2 is a single-strand RNA virus that interacts with host cells through four structural proteins: spike (S), membrane (M), envelope (E), and nucleocapsid (N). The S proteins form spikes on the surface of the virus that bind primarily to the angiotensin-converting enzyme 2 (ACE2) receptor protein on lung cells of the host, engaging a mechanism that allows the virus to penetrate the host cell membrane and release its genetic material. Once inside the host cell, other structural proteins of the virus support the process of viral replication.4 These structural proteins are the primary target of a multi-staged immune response, which plays out over several days, and variations in this response can result in different disease severities, ranging from asymptomatic infection to death (Figure 1).
The start of infection triggers an initial response by the innate immune system,5 an immediate line of defense that over the course of approximately the first 3 days engages inflammatory cells such as macrophages to neutralize virus particles. Early release of the protein interferon also helps to initiate the adaptive immune system,6 which conditions the long-term response and begins to function 1 to 2 weeks after infection. B cells are activated, producing antibodies that bind to the primary structural proteins of the virus, and T cells are formed that destroy virus particles and kill infected cells before they can release virus.
SARS-CoV-2 has adaptations that enable it to evade the initial response of the innate immune system, allowing the virus to replicate unhindered prior to activation of the adaptive immune system, which must be trained to recognize the virus constituents. Clinically severe variations of the disease often occur when the viral load quickly becomes too large and the adaptive immune response is insufficient to control the infection.
COVID-19 Vaccine Approaches and Results
COVID-19 vaccines currently in phase 3 trials or approved either fully or on an emergency basis use a range of mechanisms to train the immune system to recognize and destroy SARS-CoV-2. These approaches are summarized below, with specific characteristics listed in Table 1.7-25
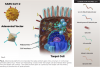
Vaccines based on dead SARS-CoV-2 viruses: Vaccines based on killed viruses produce an immune response that is based on recognition of the complete set of antigens that present on the virus (Figure 2). These viruses cannot replicate in vivo, and thus produce a shorter-lived immune response than vaccines based on live unattenuated virus. Vaccines based on dead viruses are often injected with an adjuvant to boost the immune response. Compared to other approaches, vaccines based on dead virus are inexpensive and relatively easy to store and handle, making them more suitable to less developed environments. Several vaccines based on killed virus are available on an emergency basis.
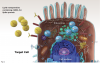
Vaccines based on messenger RNA (mRNA): mRNA coding for the spike protein is encapsulated within lipid nanoparticles (Figure 3). These enter the cell and release the mRNA, diffusing within the cytoplasm into the ribosomes, which produce the spike protein. The spike proteins may then be expressed on the cell surface, or fragments of the protein may end up traveling through the circulatory system. Presence of the spike protein elicits a powerful immune response. Two doses of the vaccine are required for full immune response, with the second dose 2 to 3 weeks after the first injection. Because mRNA is unstable at room temperature or under refrigeration, these vaccines must be stored in low-temperature freezers.
Currently, two mRNA vaccines have completed phase 3 trials and have received emergency use authorization: BNT162b2 (Pfizer/BioNTech, United States/Germany) and mRNA-1273 (Moderna, United States). At the time of this writing, these two mRNA vaccines and the Johnson and Johnson adenoviral vaccine were the only vaccines approved for emergency use in the United States.
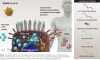
Vaccines based on adenoviral vectors: In this approach, called transfection, non-replicating adenoviral vectors are used to infect cells and deliver DNA sequences coding for the spike protein into the cell nucleus (Figure 4). Simian (nonhuman primate) adenoviral vectors are often chosen because they are not recognized by the human immune system. The transfected gene sequences coding for the spike protein is then copied and translated, resulting in distribution of the antigen on the cell surface and as fragments in the cell circulatory system. Examples of this type of platform have gone through phase 3 trials and are being used on an emergency basis. Adenoviral vaccines currently in emergency usage include those developed by Johnson and Johnson and Sputnik, based on modified human adenoviruses, and AstraZeneca, based on a chimpanzee adenovirus. These vaccines can be administered in one dose and require only refrigeration for storage.
Spike protein-based vaccines: A US company, Novavax, is conducting phase 3 trials of a vaccine based on a recombinant spike protein. The vaccine is synthesized by transfecting moth cells with a gene sequence coding for the spike protein introduced with a baculoviral vector (Figure 5). The spike proteins are then expressed on the surface of the cells, harvested, and formed into coronavirus-shaped nanoparticles studded with spike proteins. Phase 3 clinical trials have been completed in the United Kingdom and South Africa, with studies in the United States and Mexico currently underway.
Vaccines and COVID-19 Variants
At the time of this writing, three COVID-19 vaccines are approved in the United States, and as of March 8, 2021, approximately 25 million Americans were fully vaccinated and more than 50 million had received their first dose. With approximately 2 million injections occurring per day, it is plausible that well over two-thirds of the US population will have some immunity to COVID-19 by mid-summer 2021. However, the potential of this progress in restoring normal life may be threatened by the ongoing mutation of the SARS-CoV-2 virus, a process that occurs naturally in viruses and is accelerated under pandemic conditions.
Because COVID-19 vaccines stimulate an immune reaction against the spike protein, mutations that alter that protein may reduce the efficacy against those variants. The best-known variants emerging over the past year have been the UK (B1.1.7), South African (B1.351), and Brazilian (P.1) variants. Recent laboratory studies have tended to support the efficacy of vaccines against these variants. One study (letter to the editor) found that sera from subjects treated with the Pfizer BNT162b2 mRNA vaccine showed strong neutralizing activity against all of these variants.26 Another study currently in preprint form showed that exposure to the new variants did not alter the reactivity of CD4+ and CD8+ T cells derived from subjects treated with the Moderna and Pfizer mRNA vaccines.27 While phase 3 trials have tended to show decreased protection for the Novavax25 and Johnson and Johnson vaccines21 against mild infections for the UK and South African population, both of these vaccines fully protected against hospitalization and death. Although the South African variant has been detected in the United States, thus far it has not spread rapidly. As long as the pace of vaccinations in the United States continues its rapid increase, and the rate of new vaccinations exceeds the rate of new infections, experts do not consider it likely that the South African or Brazilian variants constitute a serious threat to the efficacy of vaccines in reducing COVID-19 in the United States.
Transmission of COVID-19 by Vaccinated Individuals
While clinical trials have shown that multiple vaccines reduce the risk for contracting symptomatic COVID-19, the risk of a vaccinated person contracting an asymptomatic case of COVID-19 and then transmitting it to an unvaccinated person has not been as well established until recently. Recent observational studies that monitored large numbers of subjects have indicated that the mRNA vaccines reduce COVID-19 infections as determined by polymerase chain reaction (PCR). A study of nearly 600,000 Israelis enrolled in a large national healthcare organization found that two doses of the Pfizer mRNA vaccine provided 92% efficacy against COVID-19 infection as determined by PCR testing.28 A multicenter study in the United States of nearly 4,000 healthcare workers and first responders continuously monitored by PCR testing found that vaccination with two doses of the mRNA vaccines resulted in a 90% efficacy against any COVID-19 infection.29 These results agree with other recent reports from the United States,30 Israel,31 and the United Kingdom,32 indicating substantial reductions of PCR-defined COVID-19 infection rate in healthcare workers. These results are consistent with the idea that IgG antibodies, which are produced by COVID-19 vaccines, are known to densely populate the nasal mucosa.33 Additionally, studies demonstrate that systemic vaccines produce high levels of nasal immunoglobin IgA.34
Thus, a vaccinated person exposed to SARS-CoV-2 would probably have a reduced viral load in their nose and throat, and would be less likely to infect other people. So, while transmission of COVID-19 by vaccinated people is possible, the risk appears to be low based on evidence gathered thus far.
Conclusion
In conclusion, roughly 1 year after the emergence of the COVID-19 pandemic, the unprecedented response of the scientific community has resulted in 21 vaccines that are currently in phase 3 clinical trials or being used on an emergency basis in many countries. These vaccines use a range of different approaches, from traditional (inactivated virus) to novel (mRNA vaccines). They are extremely effective in reducing the risk of moderate to severe cases of COVID-19 and preventing hospitalization and death. The vaccines show varying degrees of effectiveness at preventing all levels of COVID-19 infections, including asymptomatic ones, and early data appear to show that they reduce transmission as well as infection rates. Some emerging variants, such as the B1.351 variant in South Africa, appear to reduce the efficacy of some of the vaccines, although they are still effective enough to be considered useful. Fortunately, the design of some of the novel mRNA, adenoviral vector, and protein vaccines reduce the complexity of modifying these agents to counteract new variants. For countries such as the United States, the ability to use these new vaccines to restore normality will depend on the ability to vaccinate the population more rapidly than the disease is currently spreading, while maintaining the infrastructure to rapidly develop and distribute boosters to defeat emerging variants.
About the Authors
Thomas F. Lang, PhD Professor and Associate Dean for Research Emeritus (Dentistry), Department of Radiology and Biomedical Imaging, School of Medicine, University of California San Francisco, San Francisco, California
Robert M. Eber, DDS, MS Clinical Professor and Director of Clinical Research, Departments of Periodontology and Oral Medicine, School of Dentistry, University of Michigan, Ann Arbor, Michigan
Ramneek Rai, DDS Director of Health and Safety and Assistant Clinical Professor,Department of Preventive and Restorative Dental Sciences, School of Dentistry, University of California San Francisco, San Francisco, California
Michael S. Reddy, DMD, DMSc Dean and Associate Vice Chancellor, Department of Orofacial Sciences, School of Dentistry, University of California San Francisco, San Francisco, California
Queries to the author regarding this course may be submitted to authorqueries@aegiscomm.com.
References
1. World Health Organization. Coronavirus disease (COVID-19) pandemic. WHO website. Updated April 22, 2021. https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed April 22, 2021.
2. Centers for Disease Control and Prevention. COVID Data Tracker. CDC website. Updated April 22, 2021. https://covid.cdc.gov/covid-data-tracker/#datatracker-home. Accessed April 22, 2021.
3. Singh R, Kang A, Luo X, et al. COVID-19: Current knowledge in clinical features, immunological responses, and vaccine development. FASEB J.2021;35(3):e21409.
4. Mariano G, Farthing RJ, Lale-Farjat SLM, Bergeron JRC. Structural characterization of SARS-CoV-2: where we are, and where we need to be. Front Mol Biosci. 2020;7:605236.
5. Vabret N, Britton GJ, Gruber C, et al. Immunology of COVID-19: current state of the science. Immunity. 2020;52(6):910-941.
6. Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371(6529):eabf4063.
7. Xia S, Zhang Y, Wang Y, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2021;21(1):39-51.
8. Sinopharm. China grants conditional market approval for Sinopharm CNBG's COVID-19 Vaccine. Sinopharm website. January 2, 2021. http://www.sinopharm.com/en/s/1395-4173-38862.html. Accessed April 22, 2021.
9. Wu Z, Hu Y, Xu M, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021; S1473-3099 (20)30987-7.
10. Sinovac. Sinovac Announces Phase III Results of Its COVID-19 Vaccine. Sinovac website. February 5, 2021. http://www.sinovac.com/?optionid=754auto_id=922. Accessed April 22, 2021.
11. Ella R, Vadrevu KM, Jogdand H, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: a double-blind, randomised, phase 1 trial. Lancet Infect Dis. 2021;S1473-3099(20)30942-7.
12. Bharat Biotech. Bharat Biotech Announces Phase 3 Results of COVAXIN®: India's First COVID-19 Vaccine Demonstrates Interim Clinical Efficacy of 81%. Bharat Biotech website. March 3, 2021. https://www.bharatbiotech.com/images/press/covaxin-phase3-efficacy-results.pdf. Accessed April 22, 2021.
13. Corbett KS, Flynn B, Foulds KE, et al. Evaluation of the mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates. N Engl J Med. 2020; 383(16):1544-1555.
14. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403-416.
15. Vogel AB, Kanevsky I, Che Y, et al. BNT162b vaccines protect rhesus macaques from SARS-CoV-2. Nature. 2021;592(7853):283-289.
16. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27): 2603-2615.
17. Ewer KJ, Barrett JR, Belij-Rammerstorfer S, et al. T cell and antibody responses induced by a single dose of ChAdOx1 nCoV-19 (AZD1222) vaccine in a phase 1/2 clinical trial. Nat Med. 2021;27(2):270-278.
18. Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99-111.
19. Logunov DY, Dolzhikova IV, Shcheblyakov DV, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397(10275):671-681.
20. Mercado NB, Zahn R, Wegmann F, et al. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature. 2020;586(7830):583-588.
21. Food and Drug Administration. Vaccines and Related Biological Products Advisory Committee Meeting, February 26, 2021. FDA Briefing Document. Janssen Ad26.COV2.S Vaccine for the Prevention of COVID-19. 2021. https://www.fda.gov/media/146217/download. Accessed April 22, 2021.
22. Wu S, Zhong G, Zhang J, et al. A single dose of an adenovirus-vectored vaccine provides protection against SARS-CoV-2 challenge. Nat Commun. 2020;11(1):4081.
23. CanSinoBIO. NMPA Accepts the Application for Conditional Marketing Authorization of CanSinoBIO's COVID-19 Vaccine Convidecia™. CanSinoBIO website. February 24, 2021. http://www.cansinotech.com/html/1///179/180/651.html. Accessed April 22, 2021.
24. Tian JH, Patel N, Haupt R, et al. SARS-CoV-2 spike glycoprotein vaccine candidate NVX-CoV2373 immunogenicity in baboons and protection in mice. Nat Commun. 2021;12(1):372.
25. Novavax. Novavax Confirms High Levels of Efficacy Against Original and Variant COVID-19 Strains in United Kingdom and South Africa Trials. Novavax website. March 11, 2021. https://ir.novavax.com/news-releases/news-release-details/novavax-confirms-high-levels-efficacy-against-original-and-0. Accessed April 22, 2021.
26. Liu Y, Liu J, Xia H, et al. Neutralizing activity of BNT162b2-elicited serum. N Engl J Med. 2021. doi:10.1056/NEJMc2102017.
27. Tarke A, Sidney J, Methot N, et al. Negligible impact of SARS-CoV-2 variants on CD4 (+) and CD8 (+) T cell reactivity in COVID-19 exposed donors and vaccinees [preprint]. bioRxiv. 2021;2021.02.27.433180.
28. Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384 (15):1412-1423.
29. Thompson MG, Burgess JL, Naleway AL, et al. Interim estimates of vaccine effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines in preventing SARS-CoV-2 infection among health care personnel, first responders, and other essential and frontline workers - eight U.S. locations, December 2020-March 2021. MMWR Morb Mortal Wkly Rep. 2021;70(13):495-500.
30. Keehner J, Horton LE, Pfeffer MA, et al. SARS-CoV-2 infection after vaccination in health care workers in California. N Engl J Med. 2021. doi:10.1056/NEJMc2101927.
31. Benenson S, Oster Y, Cohen MJ, Nir-Paz R. BNT162b2 mRNA Covid-19 vaccine effectiveness among health care workers. N Engl J Med. 2021. doi:10.1056/NEJMc2101951.
32. Jones NK, Rivett L, Seaman S, et al. Single-dose BNT162b2 vaccine protects against asymptomatic SARS-CoV-2 infection. eLife. 2021;10:e68808.
33. Horton RE, Vidarsson G. Antibodies and their receptors: different potential roles in mucosal defense. Front Immunol. 2013;4:200.
34. Clements JD, Freytag LC. Parenteral vaccination can be an effective means of inducing protective mucosal responses. Clin Vaccine Immunol. 2016;23(6):438-441.