You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
Platelet concentrates (PCs) have been used to improve healing during and after medical and dental procedures for more than 50 years.1 The growth factors and regenerative potential of PCs were first identified by Ross and colleagues.1 PCs have become a topic of interest in dentistry to promote bone and soft-tissue healing. First-generation PCs, platelet-rich plasma (PRP), and second-generation platelet-rich fibrin (PRF) are valued for their properties that may stimulate healing and improve hard- and soft-tissue repair and regeneration.2-4 These materials generally contain increased concentrations of growth factors and cytokines that are present at physiologic levels in platelets in vivo. PCs also may contain biological scaffolds that can induce release of growth factors and cytokines from surrounding tissues during the healing process. These include platelet-derived growth factor (PDGF), transforming growth factor-β (TGF-β), bone morphogenetic protein-2 (BMP-2), and vascular endothelial growth factor (VEGF).2,4
There are two types of platelet concentrates used most frequently in dentistry. Platelet-rich plasma (PRP, or leukocyte-PRP [L-PRP]) is a concentrate of anticoagulated blood that consists of leukocytes and a high platelet concentration resulting from a double centrifugation. PRP preparation also requires an activator before clinical application. Platelet-rich fibrin (PRF or L-PRF) is characterized by high levels of platelets and leukocytes in a dense fibrin matrix. PRF does not require an activator before use and is prepared from whole blood after a single centrifugation step.
Additionally, techniques describing subsequent treatments of PCs (ie, heat or molding using templates) to improve handling characteristics or longevity have been described in the literature. Suggested indications for the use of PCs include enhancing soft- and hard-tissue regeneration, particularly as an adjunct to the use of other grafting materials.5,6 Clinical research has focused on the use of PCs to improve outcomes in the treatment of intrabony periodontal defects, furcation defects, guided bone regeneration, and gingival recession defects.2-4
Evolution of Platelet Concentrates: First- and Second-Generation PCs
Platelets are a critical component of hemostasis and wound stability. During healing after injury, platelets are a source of growth factors.7.8 During the preparation of PCs, platelets from autogenous blood release their growth factors, and these growth factors are concentrated throughout the preparation procedure.Preparation methods of PCs have evolved over time with changes aimed at simplifying preparation protocols and producing PCs with characteristics that may be advantageous for different types of procedures.
First-Generation PCs: Platelet-Rich Plasma
PRP is an autologous biological product created from the plasma fraction of blood with a platelet concentration up to fivefold to eightfold greater than that found in whole blood.9 After PRP was initially identified as a potential source of growth factors to improve healing, it was subsequently used throughout various fields of medicine, including dermatology and orthopedics and for wound healing and oral surgical applications.10,11 PRP contains high concentration of platelets but minimal amounts of fibrinogen. Platelet α-granules released during PRP preparation contain growth factors that are active for approximately 3 to 5 days. These growth factors then induce a local proliferative phase for approximately 7 days after PRP application, which may promote healing of the wound at the site of application.12
PRP delivers cytokines and growth factors locally where it is applied. Such growth factors can enhance the migration and differentiation of osteoprogenitor cells. These growth factors may also upregulate angiogenesis during initial wound healing. PRP is biocompatible and, because it is autologous, does not convey a risk of transmissible disease.PRP preparation, however, is technique sensitive and requires additives that lead to rapid fibrin polymerization. This rapid polymerization results in a weak fibrin network and a liquid consistency. The in vitrostability of PRP and the release kinetics and activation of growth factors occurs for a limited length of time.12
Second-Generation PCs: Platelet-Rich Fibrin
PRF is a second-generation PC first developed in 2001 to be used specifically in the oral cavity.13 The incorporation of PRF during soft- and hard-tissue reconstruction of oral structures has demonstrated accelerated healing.5 PRF is also called leukocyte and platelet-rich fibrin (L-PRF), because it contains many leukocytes and has a gel-like consistency that allows it to be molded into pliable membrane and/or incorporated into other graft materials. Enhanced handling capabilities of PRF alone as well as in combination with other regenerative materials have been reported.14
PRF is produced after unaltered progressive polymerization occurs during and immediately following centrifugation. The preparation of PRF does not require additives, which decreases preparation cost and complexity. Also, PRF contains a fibrin matrix with greater numbers of leukocytes and cytokines compared with first-generation PCs. Because of this, the structural integrity of PRF generally results in a gel or membrane-like consistency.6 PRF's fibrin matrix may result in more gradual and sustained release of growth factors and matrix glycoproteins, better mirroring natural healing processes.7 This structural integrity also allows for the use of PRF as a surrogate clot or a membrane/matrix.15 The combination of PRF with graft materials has been reported to improve handling capabilities and retain volumetric stability.7,14 Due to the natural polymerization process, the quantity of PRF that results from a blood draw may be variable and PRF must be prepared shortly before it is used clinically because dehydration can occur and lead to shrinkage or loss of structural integrity during storage.15 Furthermore, the presence of leukocytes may alter PRF's biologic properties by enhancing immune and antimicrobial function and possibly aiding in the regulation of growth factors at the wound site.6,16
A brief summary comparing and contrasting PRP and PRF is presented in Table 1. The preparation steps for first- and second-generation PCs are illustrated in Figure 1.
Clinical Implications of PCs in Dentistry
PCs are currently used throughout dentistry, particularly in periodontal and oral surgical treatments. PCs have demonstrated enhanced wound healing after both hard- and soft-tissue grafting, and their use has been associated with improved patient-centered outcomes, including decreased postoperative pain.17-20 Furthermore, additional novel preparations using heat treatment of plasma have been found to extend the working properties of PRF from 2 to 3 weeks to 4 to 6 months, which may be advantageous in certain clinical scenarios,including at sites where prolonged healing may be anticipated, such as large areas of hard-tissue grafting.21
Effects of PCs on Cells in Oral Tissue Regeneration
Growth factors released by PCs play an important role in wound healing and include PDGF, TGF-β, VEGF, epithelial growth factor (EGF), and insulin-like growth factor (IGF-1), as well as basic fibroblast growth factor (bFGF) and blood proteins known to act as cell adhesion molecules that promote osteoconduction.5,7,10,12 In vitrostudies evaluating the effects of PCs on individual oral cell types have demonstrated variable outcomes regarding the proliferation and differentiation of fibroblasts and osteoblasts.10,22-24 PRF has demonstrated inhibition of osteoprotegerin and upregulation of alkaline phosphatase expression, which may indicate that PRF could induce differentiation of periodontal ligament-derived stem cells to osteoblasts.25
Another advantage of PCs may be potential antimicrobial properties; PRP and PRF have demonstrated in vitroinhibition of bacterial growth of both Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans.26 Research also suggests that PCs may suppress long-term expression of proinflammatory cytokines and therefore limit local and systemic effects of chronic inflammation.27
Adjunctive Use of PCs in Hard-Tissue Regeneration
The use of PCs to enhance hard-tissue grafting has been associated with a decreased need for bone graft material, faster healing, and increased new bone formation in both guided bone regeneration (GBR) and periodontal guided tissue regeneration (GTR) applications compared to grafts without PCs.5,18,28 Specifically, the adjunctive use of PCs with bone replacement grafting during maxillary sinus augmentation has been associated with increased histologic bone formation, bone maturity, and radiographic bone formation when used in combination with demineralized freeze-dried bone allograft (FDBA), but not when used with acellular bovine bone matrix (ABBM).16,29-32
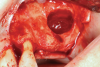
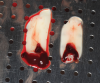
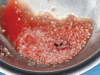
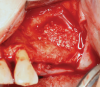
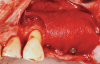
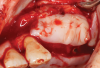
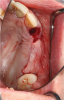
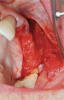
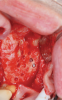
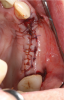
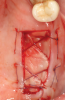
Membranes formed from PRF have also been used during sinus augmentation procedures to repair perforations of the Schneiderian membrane33 and to close a lateral window (Figure 2 through Figure 7).34 In such cases, clinical results were similar to those seen when resorbable collagen barrier membranes were used.35 When used in combination with bone grafts for ridge augmentation and socket grafting procedures, PCs demonstrated increased radiographic bone density, increased vital bone percentage, and earlier bone formation and maturation (Figure 8 through Figure 14).36 Adjunctive use of PCs with bone replacement grafts have also demonstrated improved regenerative outcomes in intrabony defects when compared with bone graft alone.28 However, PRF, but not PRP, demonstrated additive clinical benefits when used with GTR.37
An additional and often-cited advantage of the use of PCs is the improvement in handling of other graft materials when they are used in combination.14,36 Many case reports indicate that PCs may improve handling capabilities, postoperative discomfort, and healing of overlying soft tissues, which may indicate their clinical use for assisting practitioners in delivering optimal outcomes.14,36
Adjunctive Use of PCs in Soft-Tissue Regeneration
Adjunctive application of PCs during soft-tissue grafting has resulted in greater postoperative root coverage with coronally advanced flap (CAF) alone, but not CAF and connective tissue graft (CTG).38 Sites treated with root coverage procedures and use of adjunctive PCs have also been shown to demonstrate superior gingiva index and an increase in gingival thickness as compared to such procedures without PCs.39 Although the ultimate root coverage benefits of PCs may be equivocal, it has been proposed that PCs may aid postoperative pain control and more rapid healing.40,41
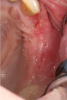
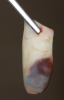
Another clinical scenario in which the use of PCs may be beneficial during soft-tissue grafting procedures is the use of PRF as a biologic bandage at palatal donor sites where CTG or free gingival graft (FGG) harvest has occurred. PRF application to a palatal donor site (Figure 15 and Figure 16) resulted in faster healing and nearly complete wound epithelialization 1-week postoperatively with minimal clinical inflammation compared to incomplete wound closure 2 weeks postoperatively where PRF was not used.42
Conclusion
Platelet concentrates are autologous graft materials that have potential uses as adjuncts during oral regeneration procedures. The different types of PCs and evolving preparation techniques have created a spectrum of biomaterials available to practitioners that allows for the selection of preferred materials for individual clinical situations. Standardization of preparation and research protocols is needed to further identify optimal clinical scenarios for the use of PCs in oral regeneration, and well-designed future studies that focus on the clinical effectiveness of PCs on soft- and hard-tissue grafting will help further clarify the use of these materials.
About the Authors
Maria L. Geisinger, DDS, MS
Professor, Director, Advanced Education in Periodontology, Department of Periodontology, School of Dentistry, University of Alabama at Birmingham, Birmingham, Alabama
Peter Nasseh, DDS
Resident, Department of Periodontology, School of Dentistry, University of Alabama at Birmingham, Birmingham, Alabama
Geisy Galviz, DDS
Resident, Department of Periodontology, School of Dentistry, University of Alabama at Birmingham, Birmingham, Alabama
Karen Y. Jo, DDS
Resident, Department of Periodontology, School of Dentistry, University of Alabama at Birmingham, Birmingham, Alabama
Anthony M. Pikos, DDS, MS
Private Practice limited to Periodontics, Tampa, Florida
Queries to the author regarding this course may be submitted to authorqueries@aegiscomm.com.
References
1. Ross R, Glomset J, Kariya B, Harker L. A platelet-dependent serum factor that stimulates the proliferation of arterial smooth muscle cells in vitro. Proc Natl Acad Sci U S A. 1974;71(4):1207-1210.
2. Feigin K, Shope B. Use of platelet-rich plasma and platelet-rich fibrin in dentistry and oral surgery: introduction and review of the literature. J Vet Dent. 2019;36(2):109-123.
3. Ghanaati S, Herrera-Vizcaino C, Al-Maawi S, et al. Fifteen years of platelet rich fibrin in dentistry and oromaxillofacial surgery: how high is the level of scientific evidence? J Oral Implantol. 2018;44(6):471-492.
4. Fujioka-Kobayashi M, Miron RJ, Hernandez M, et al. Optimized platelet-rich fibrin with the low-speed concept: growth factor release, biocompatibility, and cellular response. J Periodontol.2017;88(1):112-121.
5. Dohan DM, Choukroun J, Diss A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I: technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):e37-e44.
6. Dohan DM, Choukroun J, Diss A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part II: platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):e45-e50.
7. Dohan Ehrenfest DM, de Peppo GM, Doglioli P, Sammartino G. Slow release of growth factors and thrombospondin-1 in Choukroun's platelet-rich fibrin (PRF): a gold standard to achieve for all surgical platelet concentrates technologies. Growth Factors. 2009;27(1):63-69.
8. Nikolidakis D, Jansen JA. The biology of platelet-rich plasma and its application in oral surgery: literature review. Tissue Eng Part B Rev. 2008;14(3):249-258.
9. Lee JW, Kwon OH, Kim TK, et al. Platelet-rich plasma: quantitative assessment of growth factor levels and comparative analysis of activated and inactivated groups. Arch Plast Surg. 2013;40(5):530-535.
10. Tsai CH, Shen SY, Zhao JH, Chang YC. Platelet-rich fibrin modulates cell proliferation of human periodontally related cells in vitro. J Dent Sci. 2009;4(3):130-135.
11. Li ZJ, Choi HI, Choi DK, et al. Autologous platelet-rich plasma: a potential therapeutic tool for promoting hair growth. Dermatol Surg. 2012;38(7 Pt 1):1040-1046.
12. Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. 2009;27(3):158-167.
13. Choukroun J, Adda F, Schoeffler C, Vervelle A. Une opportunité en paro-implantologie: Le PRF. Implantodontie. 2001;42:55-62.
14. Saluja H, Dehane V, Mahindra U. Platelet-rich fibrin: A second generation platelet concentrate and a new friend of oral and maxillofacial surgeons. Ann Maxillofac Surg. 2011;1(1):53-57.
15. Khorshidi H, Raoofi S, Bagheri R, Banihashemi H. Comparison of the mechanical properties of early leukocyte- and platelet-rich fibrin versus PRGF/endoret membranes. Int J Dent. 2016;2016:1849207.
16. Bielecki T, Dohan Ehrenfest DM, Everts PA, Wiczkowski A. The role of leukocytes from L-PRP/L-PRF in wound healing and immune defense: new perspectives. Curr Pharm Biotechnol. 2012;13(7):1153-1162.
17. Choukroun J, Diss A, Simonpieri A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part IV: clinical effects on tissue healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):e56-e60.
18. Panda S, Doraiswamy J, Malaiappan S, et al. Additive effect of autologous platelet concentrates in treatment of intrabony defects: a systematic review and meta-analysis. J Investig Clin Dent. 2016;7(1):13-26.
19. Miron RJ, Zucchelli G, Pikos MA, et al. Use of platelet-rich fibrin in regenerative dentistry: a systematic review. Clin Oral Investig. 2017;21(6):1913-1927.
20. Luo HY, Li RM, Wang CL, et al. The adjunctive use of platelet concentrates in the therapy of gingival recessions: a systematic review and meta-analysis. J Oral Rehabil. 2015;42(7):552-561.
21. Kobayashi E, Flückiger L, Fujioka-Kobayashi M, et al. Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clin Oral Investig. 2016;20(9):2353-2360.
22. Krasna M, Domanović D, Tomsic A, et al. Platelet gel stimulates proliferation of human dermal fibroblasts in vitro. Acta Dermatovenerol Alp Pannonica Adriat. 2007;16(3):105-110.
23. Uggeri J, Belletti S, Guizzardi S, et al. Dose-dependent effects of platelet gel releasate on activities of human osteoblasts. J Periodontol. 2007;78(10):1985-1991.
24. Cenni E, Ciapetti G, Pagani S, et al. Effects of activated platelet concentrates on human primary cultures of fibroblasts and osteoblasts. J Periodontol. 2005;76(3):323-328.
25. Chang YC, Zhao JH. Effects of platelet-rich fibrin on human periodontal ligament fibroblasts and application for periodontal infrabony defects. Aust Dent J. 2011;56:365-371.
26. Yang LC, Hu SW, Yan M, et al. Antimicrobial activity of platelet-rich plasma and other plasma preparations against periodontal pathogens. J Periodontol. 2015;86:310-318.
27. El-Sharkawy H, Kantarci A, Deady J, et al. Platelet-rich plasma: growth factors and pro- and anti-inflammatory properties. J Periodontol.
2007;78:661-669.
28. Carlson NE, Roach RB Jr. Platelet-rich plasma: clinical applications in dentistry. J Am Dent Assoc. 2002;133(10):1383-1386. doi:10.14219/jada.archive.2002.0054
29. Ali S, Bakry SA, Abd-Elhakam H. Platelet-rich fibrin in maxillary sinus augmentation: a systematic review. J Oral Implantol. 2015;41(6):746-753.
30. Inchingolo F, Tatullo M, Marrelli M, et al. Trial with platelet-rich fibrin and Bio-Oss used as grafting materials in the treatment of the severe maxillar bone atrophy: clinical and radiological evaluations. Eur Rev Med Pharmacol Sci. 2010;14(12):1075-1084.
31. Zhang Y, Tangl S, Huber CD, et al. Effects of Choukroun's platelet-rich fibrin on bone regeneration in combination with deproteinized bovine bone mineral in maxillary sinus augmentation: a histological and histomorphometric study. J Craniomaxillofac Surg. 2012;40(4):321-328.
32. Tatullo M, Marrelli M, Cassetta M, et al. Platelet-rich fibrin (P.R.F.) in reconstructive surgery of atrophied maxillary bones: clinical and histological evaluations. Int J Med Sci. 2012;9(10):872-880.
33. Chia-Lai PJ, Orlowska A, Al-Maawi S, et al. Sugar-based collagen membrane cross-linking increases barrier capacity of membranes. Clin Oral Investig. 2018;22(4):1851-1863.
34. Bosshardt DD, Bornstein MM, Carrel JP, et al. Maxillary sinus grafting with a synthetic, nanocrystalline hydroxyapatite-silica gel in humans: histologic and histomorphometric results. Int J Periodontics Restorative Dent. 2014;34(2):259-267.
35. Gassling V, Purcz N, Braesen JH, et al. Comparison of two different absorbable membranes for the coverage of lateral osteotomy sites in maxillary sinus augmentation: a preliminary study. J Craniomaxillofac Surg. 2013;41(1):76-82.
36. Del Fabbro M, Bortolin M, Taschieri S. Is autologous platelet concentrate beneficial for post-extraction socket healing? A systematic review. Int J Oral Maxillofac Surg. 2011;40(9):891-900.
37. Gupta SJ, Jhingran R, Gupta V, et al. Efficacy of platelet-rich fibrin vs. enamel matrix derivative in the treatment of periodontal intrabony defects: a clinical and cone beam computed tomography study. J Int Acad Periodontol. 2014;16(3):86-96.
38. Cheung WS, Griffin TJ. A comparative study of root coverage with connective tissue and platelet concentrate grafts: 8-month results. J Periodontol. 2004;75(12):1678-1687.
39. Huang LH, Neiva REF, Soehren SE, et al. The effect of platelet-rich plasma on the coronally advanced flap root coverage procedure: a pilot human trial. J Periodontol. 2005;76(10):1768-1777.
40. Jankovic S, Aleksic Z, Klokkevold P, et al. Use of platelet-rich fibrin membrane following treatment of gingival recession: a randomized clinical trial. Int J Periodontics Restorative Dent.2012;32(2):e41-e50.
41. Jankovic S, Aleksic Z, Milinkovic I, Dimitrijevic B. The coronally advanced flap in combination with platelet-rich fibrin (PRF) and enamel matrix derivative in the treatment of gingival recession: a comparative study. Eur J Esthet Dent. 2010;5(3):260-273.
42. Kulkarni M, Thomas B, Varghese J, Bhat G. Platelet-rich fibrin as an adjunct to palatal wound healing after harvesting a free gingival graft: a case series. J Indian Soc Periodontol.2014;18(3):399-402.