You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
Vaping, or electronic cigarette usage, is a trendy phenomenon, particularly among youth. Various electronic devices are widely available that deliver nicotine in an alternative way compared to conventional cigarettes while providing an authentic smoking experience physically, psychologically, and socially.1 Such devices are officially termed electronic nicotine delivery systems (ENDS), while the act of inhaling vapors is correctly known as vaping.2
An electronic cigarette (e-cig) consists of three parts: a battery, heating component, and plastic cartridge that contains a nicotine solution.1 The battery provides the heating element with the power required to vaporize or aerosolize the nicotine solution. The water vapor or aerosol produced is then inhaled by the user.2 This water vapor or aerosol is generated with a minimal combustion reaction that is thought to be less harmful to the human body than cigarette smoking and carries nicotine particles from the solution to the lungs. The e-cig industry was estimated to be worth $10 billion by 2017 and is projected to overshadow the trade of traditional tobacco before 2030.3
The History and Appeal of E-Cigarettes
Since their inception, three generations of e-cigs have been produced.4 The first generation of e-cigs was called "cig-a-like" due to their similarity in size and shape to conventional cigarettes. Unlike the first generation, the second generation consisted of two parts: the tank and the battery. The second generation also had such distinct features as a comparatively large size, sleek shape, rechargeable battery, and refillable tank. Third-generation e-cigs are a modified version of the previous generation and are known as modification e-cigs or mods. Mods include novel characteristics such as providing user control over the amount of liquid contained inside the tank and the device voltage power.4 E-cig solutions commonly contain nicotine, propylene glycol, glycerol, cannabinoid (CBD) oils, additives, and distilled water.5 Different flavors like chocolate, fruit, mint, and candy are available. Some solutions are nicotine-free while others contain up to 24 mg. Notably, labeling on these liquids is not always accurate or subject to regulation, particularly when sourced outside of official vendors.5
Generally, the use of ENDS appears to be inspired by the availability of appealing flavors, social pressure, ease of access, heavy marketing, and their widespread utilization for smoking cessation.5 The National Health Survey in 2014 revealed that 12.6% of adults had used an e-cig at least once in their lifetime.6 The survey also showed that 3.7% of adults were currently using e-cigs and that more than 90% of users continued to smoke cigarettes, which implies that e-cigs had no meaningful contribution to smoking cessation. In a younger population, the National Youth Survey reported that in 2019, compared to 2011, the use of cigarettes among middle and high school students had declined by 2.3% and 5.8%, respectively.7 At the same time, a significant increase in the use of e-cigs of 10.5% and 27.5%, respectively, had been recorded. In 2016, the US Food and Drug Administration (FDA) and Centers for Disease Control and Prevention (CDC) further revealed that more than 5 million young people had consumed e-cigs during the past 30 days, while 1 million active users had been recorded per day.8
Given the notable increase in electronic cigarette use, the objective of this article was to review the available evidence on the impacts of these devices on systemic and oral health, with particular attention given to periodontal tissue, dental caries, oral lesions, and orofacial injuries. This review will serve as a reference for clinicians across dentistry to use in assessment of patients using ENDS.
Systemic Risk of E-Cigarettes
Current evidence on the systemic risk of e-cigs is not adequate to suggest that they are safer than traditional cigarettes, although this claim is frequently made.9 Some studies have compared the aerosol produced by ENDS to cigarette smoke and found lower concentrations of carcinogenic compounds such as formaldehyde and nitrosamines.10,11 However, a significant concentration of toxic substances (acrolein, toluene, aluminum, cadmium, chromium, copper, lead, magnesium, manganese, nickel, and zinc) were observed in the water vapor.12 In addition, as with tobacco smoke, e-cig aerosol contains reactive oxygen species (ROS) and free radicals that can cause oxidative stress, affect antioxidant mechanisms, and stimulate DNA changes.13 These compounds result in increased secretion of pro-inflammatory cytokines, such as interleukin (IL)-6 and IL-8. Additionally, stress and morphological changes have been noted within human lung fibroblasts exposed to these carcinogenic compounds.
Nicotine is also reported to play a role in the development of cardiopulmonary disease and neurological disorders. For example, it can cause increased heart rate, hypertension, chronic obstructive pulmonary disease, and addiction.4,14 Research has suggested, however, that even nicotine-free ENDS can trigger inflammation and cardiovascular diseases through aerosolized ultrafine particles.4 These tiny particles are biologically active and can stimulate cardiovascular inflammation and unwanted health consequences. Another main concern of nicotine use is as a potential gateway to combustible cigarettes through addiction, keeping users in the cycle of smoking by switching between different products.5
Evidence for the negative impact of e-cigs on the lungs is growing.15 Tetrahydrocannabinol (THC), which gives users a "high" feeling, has been connected to many cases of e-cig or vaping product-associated lung injury (EVALI). Usually, users with EVALI present to hospitals with dizziness, headaches, fevers, and/or pulmonary symptoms such as coughing, difficulty breathing, shortness of breath, and chest pain. They also may present with gastrointestinal symptoms such as nausea, vomiting, and diarrhea. Vitamin E acetate, which may be added to ENDS to give the solutions a longer shelf-life, also has been linked to the current rise in EVALI.
The pathologic mechanism behind EVALI is not well understood, however two hypotheses have been proposed.16 First, toxic chemicals such as vitamin E may cause direct damage to the epithelial and endothelial alveolar cells. The other hypothesis suggests that aerosol components such as glycerine and propylene glycol may trigger the inflammatory process by changing the alveolar lung homeostasis, causing hypersensitivity to materials that are usually not harmful. According to this theory, immune cells trigger inflammation, damage, and necrosis.
EVALI is characterized by the destruction of both epithelial and endothelial alveolar cells in the lungs. The integrity of the alveolar-capillary membrane becomes damaged, causing alveolar edema, neutrophil recruitment, and cytokine involvement.17 Neutrophils are primarily responsible for this pathological reaction, which makes it comparable to acute lung injury.18 The clinical presentation of EVALI is also almost identical to that in coronavirus disease-19 (COVID-19). Individuals suffering from COVID-19 usually manifest significant pulmonary symptoms (eg, acute lung injury) that may eventually progress to acute respiratory distress syndrome and hypoxic respiratory failure. However, a major difference between the two diseases is that EVALI usually affects young individuals whereas COVID-19 is more concerning among older populations. Although conventional cigarettes likely worsen COVID-19 symptoms, it is unclear whether ENDS are associated with higher contractibility, negative progression, or poorer outcomes in COVID-19.16,19
Other e-cig ingredients have been linked to EVALI, including nicotine, propylene glycol, and diacetyl.15 Diacetyl has been shown to trigger bronchiolitis obliterans, or popcorn lung, in which bronchioles become scarred and nonfunctional, limiting their capacity to take in oxygen.20 Popcorn lung was so named after bronchiolitis obliterans was found more commonly among microwave popcorn factory workers due to chronic exposure to diacetyl's butter flavoring, which is not used anymore.
Compared to ENDS, the systemic risk of combustible cigarettes is well established. Better understanding of e-liquid components and their potential impacts on systemic health will help researchers accurately stratify risk, increase public awareness, and enable people to avoid health repercussions.
Oral Risk of e-Cigarettes
A paucity of evidence exists regarding the impacts of ENDS on oral health; however, limited studies have suggested ENDS-specific oral effects. A worldwide survey that involved 19,000 e-cig users revealed that more than half of the study population (59.8%, n = 11,000) was affected negatively by the habit, although most side effects were mild to moderate and resolved spontaneously.21 Interestingly, the most common side effect reported (including systemic effects) was dry mouth (xerostomia) (40%, n = 4,500). Additionally, 5% of participants noted mouth sores and inflammation, and 13.1% noted gingivitis.
The nicotine contained in ENDS may contribute to oral disease. About 45% of nicotine produced from the vaping process accumulates in the mouth. Additionally, the concentration of nicotine in saliva is approximately 10 times higher compared to plasma levels.22 Nicotine is a vasoconstrictor, and the resulting decrease in blood supply to the oral cavity affects migration of immune cells, tissue repair, cytoskeleton remodeling, and removal of pro-inflammatory cytokines from the gingival tissue.14 Porphyromonas gingivalis and Streptococcus mutans have also been reported to increase in gingival/periodontal tissue after nicotine use.22 ROS produced by ENDS usage may also negatively impact gingival/periodontal health. According to an in vitro study, inclusion of water vapor in human periodontal tissue leads to protein carbonylation. Subsequently, a pro-inflammatory cytokine (IL-8) is released, causing DNA damage.23
Mokeem et al examined several clinical periodontal parameters, such as plaque index, bleeding on probing, pocket depth, clinical attachment loss, radiographic marginal bone loss, whole salivary cotinine, and IL-1 and IL-6 levels in nicotine users.24 The study included 154 male subjects (39 cigarette smokers, 40 waterpipe smokers, 37 e-cig users, and 38 never-smokers). The results indicated that, clinically and radiographically, parameters of periodontal inflammation were worse among cigarette and waterpipe smokers compared with nonsmokers and e-cig users. Furthermore, bleeding on probing was less prevalent among smokers, waterpipe users, and e-cig users in comparison with nonsmokers. This is likely due to the vasoconstrictive effects of nicotine on gingival blood vessels.
In addition, Javed et al provided a questionnaire to 33 cigarette smokers, 31 e-cig users, and 30 never-smokers to compare periodontal parameters and self-perceived oral symptoms among these three groups.25 They concluded that periodontal inflammation is worse among conventional smokers compared with the other two groups. An additional study of 30 cigarette smokers, 28 e-cig users, and 31 never-smokers found that after full-mouth scaling, periodontal inflammation was worse among cigarette smokers in comparison with e-cig users and never-smokers.26 Another cross-sectional observational study assessed the effect of ENDS on the general health of 110 individuals who replaced conventional cigarettes with e-cigs.27 Participants reported a notable decrease in bacterial plaque, improvement in gingival bleeding, and better smell and taste perception. Thus, e-cig use has been claimed to be less harmful for periodontal health than cigarette smoking.
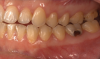
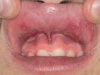
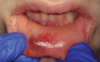
The effects of ENDS on caries activity has not been well established. A few studies suggest that vaping may increase the cariogenic potential, pointing to increased acid production from propylene glycol heating, sweet flavors, sucrose, viscous aerosol, higher bacterial attachment to tooth surface, increased biofilm formation, decreased enamel hardness, and dry mouth (Figure 1 and Figure 2).28
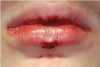
The effects of ENDS on oral mucosa are also not well studied. A pilot study comprised of a small sample of patients examined the prevalence and features of oral lesions among people who had quit smoking (n = 45) compared to e-cig users (n = 45).29 The prevalence of oral mucosal lesions was higher in those who used e-cigs (65.4%) compared to past smokers (34.6%). However, this result was not statistically significant. A case was reported on of a 15-year-old boy who had mouth ulcerations that were suggested to be secondary to marijuana vaping (Figure 3).30 His mouth ulcers disappeared and did not recur after he stopped vaping.
Current information on the potential risk of ENDS in contributing to oral dysplastic lesions and/or squamous cell carcinoma (SCC) is inadequate and arguable. A micronucleus assay test was utilized in a case control study that sampled buccal mucosae through exfoliative cytology.31 The study included 22 e-cig users, 23 current smokers, and 20 nonsmokers. The study found that e-cig use did not contribute to cellular changes, whereas micronucleus presence increased significantly with conventional smoking. Frequency of micronuclei was comparable between e-cig users and nonsmoker control groups. Micronucleus assay is commonly used to evaluate chromosomal aberrations, which suggest cellular mutagenicity. However, these promising results have several limitations, including questions regarding the use of the micronucleus assay in the oral cavity, small sample size, limited follow-up period, single site collection, and lack of blinding. Additionally, an in vitro study reported opposing results, finding that cellular changes, apoptosis, and necrosis were seen in regular epithelial cell lines and SCC lines after 48 hours to 8 weeks of exposure to e-cig aerosol. This study also was limited by various shortcomings in methodological design, such as absence of comparisons with traditional cigarette smokers, in vitro design, and a small sample.32
Bacterial diversity in the mouth prevents oral dysbiosis, a condition that can lead to gingival disease and dental decay.33 A preliminary cross-sectional study investigated saliva and buccal mucosa swabs of a human cohort that comprised 10 tobacco smokers, 10 e-cig users, and 10 control subjects and found that bacterial presence among smokers was significantly higher compared to controls and e-cig users.34 The study, however, was disadvantaged by a small sample size and short study period. Another cross-sectional study aimed to compare the presence of oral Candida albicans among 34 cigarette smokers, 33 waterpipe users, 30 e-cig users, and 32 nonsmokers. Candidawas found in 100%, 100%, 83%, and 50% of each group, respectively.35 Additionally, e-cig use might be associated with increased Candida carriage, which puts users at a higher risk for the development of oral fungal infection.36
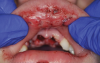
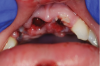
Orofacial injuries from ENDS explosions have been documented with low incidence.37 These incidents tend to occur due to excessive heating of the unit's internal lithium-ion battery. Injuries can include tooth fractures, dentoalveolar fractures, hematoma formation, traumatic ulceration, burns, and palatal perforation (Figure 4 through Figure 7). Soft-tissue damage may require cosmetic and functional surgical reconstruction.
Smoking Cessation and the Role of Dentists
Studies have shown that FDA-approved nicotine replacement therapies, such as the use of transdermal nicotine patches, nicotine gum, and nicotine lozenges, are beneficial for smoking cessation but not effective in prolonged smoking abstinence.38 However, e-cig users have been reported to remain motivated to maintain smoking abstinence because ENDS provide psychosocial benefits, helping to prevent relapse.39 Therefore, e-cigs may be considered as a less harmful alternative to combustible cigarettes in the short term.
Attempting to use e-cigs to reduce the frequency of cigarettes is not as beneficial as completely quitting smoking, however. The risk of malignancy in these patients is about the same as in those who continue smoking at baseline levels since risk appears to be connected to smoking duration, not frequency.40 In 2016, the FDA began controlling all tobacco products, including e-cigs, but FDA regulation of these products does not imply safety, and e-cigs have not been approved as a smoking cessation tool. Additionally, the regulation applies only to the liquids that contain nicotine, while nicotine-free products are not regulated and are, therefore, more readily available.41
Frequent, consistent attendance to the dental office provides dentists the opportunity to discuss their patients' concerns and needs regarding e-cig use and smoking cessation. When taking a patient's history, dentists should query about all use of tobacco and nicotine. When discussing the risks of use and cessation planning with patients, dental professionals should consider such factors as patient objectives and future plans, guidelines, and official recommendations. Equally important, patients need to understand the systemic and oral risks inherent to this habit. Oral healthcare professionals should promote FDA-approved smoking cessation methods as advocated in the American Academy of Oral Medicine Clinician's Guide.42 This accessible manual strictly states that the use of electronic devices as smoking cessation tools in the presence of various other available carcinogen-free FDA-approved smoking cessation aids is ill-advised.
Conclusion
Whether electronic cigarettes are less harmful than combustible cigarettes is debatable, as serious conflicts of interest frequently exist in published studies, particularly those funded by e-cig companies. Available evidence does suggest that e-cig use may contribute to dry mouth, dental decay, periodontal disease, and oral candidiasis. In addition, the chemicals of e-cig vapor may negatively affect DNA and cause cellular degeneration. Whether nicotine addicts benefit from using e-cigs as an alternative to smoking is still under investigation. Prolonged research with standardized parameters is required to evaluate the hazardous consequences of e-cigs on systemic and oral health.
About the Authors
Waleed Alamoudi, BDS, MSc
Oral Medicine Resident, University of Pennsylvania School of Dental Medicine, Philadelphia, Pennsylvania
Takako I. Tanaka, DDS
Director of Postdoctoral Oral Medicine Program, Professor of Clinical Oral Medicine, Department of Oral Medicine, University of Pennsylvania School of Dental Medicine, Philadelphia, Pennsylvania; Fellowship in Dental Surgery of the Royal College of Surgeons of Edinburgh (FDS RCSEd)
Eric T. Stoopler, DMD
Professor of Oral Medicine, Department of Oral Medicine, University of Pennsylvania School of Dental Medicine, Philadelphia, Pennsylvania; Fellowship in Dental Surgery of the Royal College of Surgeons of Edinburgh and England (FDSRCS); Fellowship inDental Surgery of the Royal College of Physicians and Surgeons of Glasgow (FDSRCPS)
Thomas P. Sollecito, DMD
Professor and Chairman, Department of Oral Medicine, University of Pennsylvania School of Dental Medicine, Philadelphia, Pennsylvania; Fellowship in Dental Surgery of the Royal College of Surgeons of Edinburgh (FDS RCSEd)
Katherine France, DMD, MBE
Assistant Professor of Oral Medicine, Department of Oral Medicine, University of Pennsylvania School of Dental Medicine, Philadelphia, Pennsylvania
Queries to the author regarding this course may be submitted to authorqueries@aegiscomm.com.
References
1. Glasser AM, Collins L, Pearson JL, et al. Overview of electronic nicotine delivery systems: a systematic review. Am J Prev Med. 2017;52(2):e33-e66.
2. Grana R, Benowitz N, Glantz SA. E-cigarettes: a scientific review. Circulation. 2014;129(19):1972-1986.
3. McQueen N, Partington EJ, Harrington KF, et al. Smoking cessation and electronic cigarette use among head and neck cancer patients. Otolaryngol Head Neck Surg. 2016;154(1):73-79.
4. Glantz SA, Bareham DW. E-cigarettes: use, effects on smoking, risks, and policy implications. Annu Rev Public Health. 2018;39:215-235.
5. Dinakar C, O'Connor GT. The health effects of electronic cigarettes. N Engl J Med. 2016;375(14):1372-1381.
6. Dai H, Leventhal AM. Prevalence of e-cigarette use among adults in the United States, 2014-2018. JAMA. 2019;322(18):1824-1827.
7. Cullen KA, Gentzke AS, Sawdey MD, et al. E-cigarette use among youth in the United States, 2019. JAMA. 2019;322(21):2095-2103.
8. Food and Drug Administration, HHS. Deeming tobacco products to be subject to the Federal Food, Drug, and Cosmetic Act, as amended by the Family Smoking Prevention and Tobacco Control Act; restrictions on the sale and distribution of tobacco products and required warning statements for tobacco products. Final rule. Fed Regist. 2016;81(90):28973-29106.
9. Brandon TH, Goniewicz ML, Hanna NH, et al. Electronic nicotine delivery systems: a policy statement from the American Association for Cancer Research and the American Society of Clinical Oncology. Clin Cancer Res. 2015;21(3):514-525.
10. Jensen RP, Luo W, Pankow JF, et al. Hidden formaldehyde in e-cigarette aerosols. N Engl J Med. 2015;372(4):392-394.
11. Bustamante G, Ma B, Yakovlev G, et al. Presence of the carcinogen N′-nitrosonornicotine in saliva of e-cigarette users. Chem Res Toxicol. 2018;31(8):731-738.
12. Gaur S, Agnihotri R. Health effects of trace metals in electronic cigarette aerosols-a systematic review. Biol Trace Elem Res. 2019;188(2):295-315.
13. Lerner CA, Sundar IK, Yao H, et al. Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One. 2015;10(2):e0116732.
14. Manakil J, Miliankos A, Gray M, et al. Oral health and nicotine replacement therapy product. European J Gen Dent. 2020;9(1):1-6.
15. Gotts JE, Jordt SE, McConnell R, Tarran R. What are the respiratory effects of e-cigarettes? BMJ. 2019;366:I5275.
16. Crotty Alexander LE, Bellinghausen AL, Eakin MN. What are the mechanisms underlying vaping-induced lung injury? J Clin Invest. 2020;130(6):2754-2756.
17. Johnson ER, Matthay MA. Acute lung injury: epidemiology, pathogenesis, and treatment. J Aerosol Med Pulm Drug Deliv. 2010;23(4):243-252.
18. Layden JE, Ghinai I, Pray I, et al. Pulmonary illness related to e-cigarette use in Illinois and Wisconsin. N Engl J Med. 2020;382(10):903-916.
19. Berlin I, Thomas D, Le Faou AL, Cornuz J. COVID-19 and smoking. Nicotine Tob Res. 2020;22(9):1650-1652.
20. Landman ST, Dhaliwal I, Mackenzie CA, et al. Life-threatening bronchiolitis related to electronic cigarette use in a Canadian youth. CMAJ. 2019;191(48):E1321-E1331.
21. Farsalinos KE, Romagna G, Tsiapras D, et al. Characteristics, perceived side effects and benefits of electronic cigarette use: a worldwide survey of more than 19,000 consumers. Int J Environ Res Public Health.2014;11(4):4356-4373.
22. Kumar P, Geisinger M, DeLong HR, et al. Living under a cloud: electronic cigarettes and the dental patient. J Am Dent Assoc.2020;151(3):155-158.
23. Sundar IK, Javed F, Romanos GE, Rahman I. E-cigarettes and flavorings induce inflammatory and pro-senescence responses in oral epithelial cells and periodontal fibroblasts. Oncotarget. 2016;7(47):77196-77204.
24. Mokeem SA, Alasqah MN, Michelogiannakis D, et al. Clinical and radiographic periodontal status and whole salivary cotinine, IL-1β and IL-6 levels in cigarette- and waterpipe-smokers and e-cig users. Environ Toxicol Pharmacol. 2018;61:38-43.
25. Javed F, Abduljabbar T, Vohra F, et al. Comparison of periodontal parameters and self-perceived oral symptoms among cigarette smokers, individuals vaping electronic cigarettes, and never-smokers. J Periodontol. 2017;88(10):1059-1065.
26. ALHarthi SS, BinShabaib M, Akram Z, et al. Impact of cigarette smoking and vaping on the outcome of full-mouth ultrasonic scaling among patients with gingival inflammation: a prospective study. Clin Oral Investig. 2019;23(6):2751-2758.
27. Tatullo M, Gentile S, Paduano F, et al. Crosstalk between oral and general health status in e-smokers. Medicine (Baltimore). 2016;95(49):e5589.
28. Irusa KF, Vence B, Donovan T. Potential oral health effects of e-cigarettes and vaping: a review and case reports. J Esthet Restor Dent. 2020;32(3):260-264.
29. Bardellini E, Amadori F, Conti G, Majorana A. Oral mucosal lesions in electronic cigarettes consumers versus former smokers. Acta Odontol Scand. 2018;76(3):226-228.
30. Ali NS, Billings ML, Tollefson MM, et al. Oral erosions associated with surreptitious marijuana vaping in an adolescent boy. Pediatr Dermatol. 2020;37(2):347-349.
31. Franco T, Trapasso S, Puzzo L, Allegra E. Electronic cigarette: role in the primary prevention of oral cavity cancer. Clin Med Insights Ear Nose Throat. 2016;9:7-12.
32. Yu V, Rahimy M, Korrapati A, et al. Electronic cigarettes induce DNA strand breaks and cell death independently of nicotine in cell lines. Oral Oncol. 2016;52:58-65.
33. Zhang Y, Wang X, Li H, et al. Human oral microbiota and its modulation for oral health. Biomed Pharmacother. 2018;99:883-893.
34. Stewart CJ, Auchtung TA, Ajami NJ, et al. Effects of tobacco smoke and electronic cigarette vapor exposure on the oral and gut microbiota in humans: a pilot study [erratum appears in PeerJ. 2018;6:e4693/correction-1]. PeerJ. 2018;6:e4693.
35. Mokeem SA, Abduljabbar T, Al-Kheraif AA, et al. Oral Candida carriage among cigarette- and waterpipe-smokers, and electronic cigarette users. Oral Dis. 2019;25(1):319-326.
36. Alanazi H, Semlali A, Chmielewski W, Rouabhia M. E-cigarettes increase Candida albicans growth and modulate its interaction with gingival epithelial cells. Int J Environ Res Public Health. 2019;16(2):294.
37. Rogér JM, Abayon M, Elad S, Kolokythas A. Oral trauma and tooth avulsion following explosion of e-cigarette. J Oral Maxillofac Surg. 2016;74(6):1181-1185.
38. Hartmann-Boyce J, Chepkin SC, Ye W, et al. Nicotine replacement therapy versus control for smoking cessation. Cochrane Database Syst Rev. 2018;5(5):CD000146.
39. Hajek P, Phillips-Waller A, Przulj D, et al. A randomized trial of e-cigarettes versus nicotine-replacement therapy. N Engl J Med. 2019;380(7):629-637.
40. Bahl V, Lin S, Xu N, et al. Comparison of electronic cigarette refill fluid cytotoxicity using embryonic and adult models. Reprod Toxicol. 2012;34(4):529-537.
41. Backinger CL, Meissner HI, Ashley DL. The FDA "deeming rule "and tobacco regulatory research. Tob Regul Sci. 2016;2(3):290-293.
42. Mohammad AR, Aboalela A, Sultan A, Vankevich PJ. Clinician's Guide: Tobacco Cessation. 3rd ed. Seattle, WA: American Academy of Oral Medicine; 2017.