You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
Implant restorations are distinctive in that their foundations are osseointegrated in bone and they emerge through a soft-tissue cuff into the oral cavity. When a bone-level implant is used, the abutment represents the transmucosal component of the implant-supported prosthesis. As such, this component is vulnerable to bacterial colonization that can lead to peri-implant disease.1,2 Initial colonization of peri-implant pockets with bacteria associated with periodontitis occurs within 2 weeks after implant surgery, ie, abutment connection.2 Undisturbed plaque accumulations can induce an inflammatory response after 3 weeks.3
Peri-implantitis can result in severe peri-implant bone defects.4 Peri-implantitis lesions typically are circumferential.5 In the absence of treatment, peri-implantitis appears to follow a non-linear and accelerating pattern.6 A consensus report based on the outcomes of systematic reviews estimated the prevalence of peri-implantitis to be 22%.7
In addition to being functional, implant restorations should be biocompatible, maintainable, and esthetic. Poorly constructed and inadequately delivered implant restorations can lead to peri-implant bone loss and disease. Abutment design elements, such as topography,8 materials,9 height,10 implant-abutment connection,11 and emergence profile,12 may affect the risk for developing peri-implantitis. This may be because of differences in bacterial colonization of different abutment designs, both in terms of biofilm mass and pathogenic species.
This article discusses implant abutment design elements for bone-level implants and the risk they may present for the development of peri-implant disease. This information is intended to aid the clinician in identifying risk factors with current implant restorations and improve abutment designs for future restorations.
Abutment Topography
Topography refers to the physical features of an area. Understanding what features of a structure promote the establishment of a bacterial film can be useful in minimizing its development. Bacterial adhesion to intraoral hard surfaces is influenced by the surface roughness of these structures.8 A surface roughness of R(a) = 0.2 µm has been referred to as the "thresholds R(a)." Reducing surface roughness beyond 0.2 µm has no further effect on the quantitative/qualitative microbiological adhesion or colonization.8 Polishing protocols should be used to obtain a surface roughness of R(a) = 0.2 µm for zirconia and titanium abutments.13 Extremely smooth abutments, ie, having a R(a) = 0.06 µm, showed higher probing depths and bleeding on probing.8 Therefore, the benefit of polishing an abutment surface has its limits.
While smooth abutment surfaces appear to reduce bacterial colonization, a textured abutment surface may offer the benefit of improved adherence of human fibroblasts. Laser microtextured surfaces have been shown to improve stability of the soft tissue around implant abutments.14,15 A histologic study demonstrated the ability of a laser microtextured surface to block epithelial downgrowth and provide a functional connective tissue attachment to the abutment surface.15 Intimate adherence between the connective tissue and laser-treated abutment was detected while none was found on machined abutment surfaces.15
Abutment Material
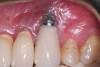
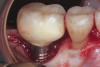
Achieving and maintaining healthy and stable peri-implant soft tissue requires the use of abutment materials that are biocompatible. This in part means using materials that have minimal affinity to bacterial colonization. Differences between titanium and zirconium abutments with regard to bacterial colonization have been of particular interest. Titanium- and zirconia-based surfaces are promptly colonized by bacterial communities similar to those found in adjacent teeth.9,16 Investigations have yielded differing results. A detailed quantitative analysis of an in vitro study found zirconia abutment surfaces have higher biofilm biomass and biofilm diversity than titanium surfaces.17 Figure 1 shows an example of an implant with a zirconia abutment with peri-implantitis.
Conversely, an in vivo study found titanium abutment surfaces to have higher total microbial counts and higher counts of pathogenic species than zirconia abutment surfaces.18 A systematic review and meta-analysis reported no statistical differences found between zirconia and titanium abutment surfaces on soft-tissue recession, probing depths, bleeding on probing, and marginal bone levels.19 The only significant difference found was a higher pink esthetic score with zirconia abutments.19
Abutment Height
Prosthetic abutment height has been defined as the distance of the crown margin to the marginal bone around the implant.10 This distance can have important consequences in marginal bone stability. In a study evaluating interproximal marginal implant bone loss at 6 and 18 months post-loading, the marginal bone loss (MBL) was found to be highest when the abutment height was less than 2 mm versus 2 mm or greater.10 The rate of MBL was non-linear. The greatest amount of interproximal MBL occurred during the first 6 months of loading as compared to the next 12 months.
A randomized clinical trial compared MBL with abutments of 1 mm and 3 mm of height on bone-level implants with a platform-switched design. The shorter abutments led to a greater amount of MBL after 6 months of healing.20 Another recent randomized clinical trial compared the use of 3 mm abutments, in combination with platform-switched implants placed 2 mm subcrestally, and the use of 1 mm abutments in implants placed equicrestally.21 This study found the implants with longer abutments had less MBL at 3, 6, and 12 months. A retrospective study compared MBL around platform-switched and non-platform-switched implants placed in maxillary areas.22 Platform-switched implants were found to have half the MBL as non-platform-switched implants with similar abutment heights.22 No difference was found between these implant sites regarding MBL around implants placed in native maxillary bone compared to grafted sites.22
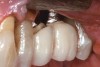
The results of these studies suggest that the shorter the prosthetic abutment height, the greater the amount of MBL that can be expected with bone-level implants (Figure 2). While these studies only measured MBL, it may be surmised that similar MBL patterns would develop on the facial and lingual aspects when the prosthetic abutment height is less than 2 mm. With greater MBL, the risk for bacterial colonization of the implant surface may increase. It would seem prudent, therefore, to place the crown margin in a position that would minimize MBL.
Implant-Abutment Connection
Proper management of the implant-abutment connection is critical to the success of an implant restoration. A comparison of healthy implant sites and those with peri-implantitis was made with regard to their clinical parameters and the microbiologic composition at the peri-implant sulcus, inside the implant-abutment connection, and at the gingival sulcus of neighboring teeth.11 As would be expected, bacterial counts were higher in all locations in the peri-implantitis group. Interestingly, the microbial counts were found to be strikingly higher inside the implant-abutment connection in the peri-implantitis group. Microleakage at the implant-abutment interface permits bacteria to enter the space inside this connection.11 This led the authors to refer to the inner portions of the implant, ie, inside the implant-abutment connection, as a reservoir for potential pathogens.11
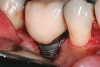
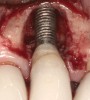
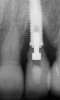
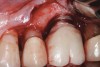
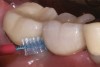
Some amount of microleakage can be expected with any current implant-abutment interface.23,24 External hexagon implants have been found to fail to prevent microleakage.23 Figure 3 and Figure 4 depict an external hexagon implant with an implant-abutment connection vulnerable to microleakage. This same study found internal hexagon implants with internal conical (Morse taper) connection to have the least amount of leakage. Zirconia abutments were found to have more microleakage than titanium abutments. As a result, this study recommended restricting the use of zirconia abutments to cases where esthetic demands are high.23
Another study, however, found titanium and zirconium abutments to have similar sealing capabilities when both had internal conical connections and increased abutment screw torque.24 Emphasis was placed on carefully following guidelines from the implant manufacturer regarding its implant components.24
Emergence Profile
The effect ofartificial crown contour on teeth and periodontal health has been studied for many years. Yuodelis et al showed that the greater the amount of facial and lingual bulge of an artificial crown, the greater the plaque accumulation at the cervical margin.25 These authors recommended undercontoured crowns for improved periodontal health. It has been argued that buccal and lingual crown contours should be "flat," not "fat."26
The emergence profile of implant-supported restorations through the soft tissues can be significantly different than that of natural teeth. The root of a natural tooth tends to gradually broaden as it meets the cementoenamel junction. Even with tapered and wide-diameter implants, the transition of implant to crown can appear abrupt. When a large crown is supported by a single implant, the prosthetic design may resemble that of a "lollipop," with the crown representing the candy and the implant being the stick. Molar sites are particularly vulnerable to this effect. Despite molars having multiple roots, often a single implant is used to support a single molar crown. The repercussions of this design may be undesirable (Figure 5).
Peri-implantitis may be the consequence of overcontoured implant restorations (Figure 6).12,27 In a recent cross-sectional radiographic analysis, the prevalence of peri-implantitis was found to be significantly greater in the bone-level group when the emergence angle was greater than 30 degrees.28 Convex profiles were found to create additional risk for peri-implantitis for bone-level implants.28 Therefore, a goal of implant placement should be to achieve an emergence profile that is straight or concave. Implants should be placed in a manner to facilitate the most ideal emergence profile. Consequently, consultation between surgical and restorative team members is advisable.
Crown Margin Position and ExcessCement Remnants
While bacteria is the etiology of peri-implant disease, excess cement remnants have been referred to as a predisposing factor (Figure 7).29 One investigation found excess cement remnants were associated with 81% of peri-implant cases evaluated.30 Other studies looked at the amount of residual excess cement remaining after cleaning of implant-supported restorations with different crown margin positions and found that the amount of excess cement remnants increased as crown margins were located more subgingivally.31,32 Also, dental radiographs were not considered to be a reliable method for detecting excess cement.32 The amount of excess cement remnants may also increase with the depth of undercut between the restoration and soft tissue.33
Clinicians must, therefore, be meticulous in their efforts to remove all excess cement remnants, especially when the crown margin is beneath the mucosal margin and when undercuts exist. The risk of the development of peri-implant disease could be lowered if the crown margin is placed at the level of the mucosal margin and sufficient access is provided for removal of excess cement.34 In non-esthetic areas, where patients may be less inclined to object, supragingival crown margins could be beneficial.
Of course, when feasible, the use of screw-retained restorations instead of cement would eliminate the risks of subgingival cement remnants.
Accessibility for Plaque Removal
Prevention of plaque accumulation is an indispensable strategy in thwarting peri-implant disease.1,7 One study found a 65% positive predictability value for peri-implantitis where there was no accessibility/capability for proper oral hygiene.35 Implants with supramucosal restoration margins showed greater therapeutic improvement when compared to those with submucosal margins.36 Lack of regular supportive therapy in patients with peri-implant mucositis was associated with increased risk for onset of peri-implantitis.7
Prosthetic reconstructions should allow for proper personal cleaning, clinical diagnosis by probing, and professional plaque removal.7,35 The provision of proper oral hygiene instructions to patients reduces the risk of peri-implant disease development.35,37 Use of a manual or powered toothbrush by the patient was found to be effective in preventative plaque control.7 Interdental oral hygiene aids, such as interdental brushes and water jets (Figure 8), ranked high for reduced gingival bleeding around teeth.37 Abutment and crown design should facilitate use of these oral hygiene aids as well as professional supportive therapies. Conversely, convex emergence profiles and interdental embrasure spaces that impede accessibility for plaque removal should be avoided.
Conclusion
Implant abutment design can play a critical role in minimizing the risk of the development of peri-implant disease. While complete consensus may not exist on all of the design elements, abutment design should promote a healthy implant site. Further study is necessary to determine the extent and specifics of the relationship between implant abutment design and peri-implant disease.
About the Author
Douglas H. Mahn, DDS
Private Practice limited to Periodontics and Implantology, Manassas, Virginia
Queries to the author regarding this course may be submitted to authorqueries@aegiscomm.com.
References
1. Caton JG, Armitage G, Berglundh T, et al. A new classification scheme for periodontal and peri-implant diseases and conditions - introduction and key changes from the 1999 classification. J Clin Periodontol. 2018;45(suppl 20):S1-S8.
2. Quirynen M, Vogels R, Peeters W, et al. Dynamics of initial subgingival colonization of ‘pristine' peri-implant pockets. Clin Oral Implants Res. 2006;17(1):25-37.
3. Zitzmann NU, Berglundh T, Marinello CP, Lindhe J. Experimental peri-implant mucositis in man. J Clin Periodontol. 2001;28(6):517-523.
4. Froum SJ, Rosen PS. A proposed classification for peri-implantitis. Int J Periodontics Restorative Dent. 2012;32(5):533-540.
5. Schwarz F, Derks J, Monje A, Wang HL. Peri-implantitis. J Periodontol. 2018;89(suppl 1):S267-S290.
6. Derks J, Schaller D, Håkansson J, et al. Peri-implantitis - onset and pattern of progression. J Clin Periodontol. 2016;43(4):383-388.
7. Jepsen S, Berglundh T, Genco R, et al. Primary prevention of peri-implantitis: managing peri-implant mucositis. J Clin Periodontol. 2015;42 suppl 16:S152-S157.
8. Bollen CM, Papaioanno W, Van Eldere J, et al. The influence of abutment surface roughness on plaque accumulation and peri-implant mucositis. Clin Oral Implants Res. 2002;7(3):201-211. doi: 10.1034/j.1600-0501.1996.070302.x.
9. de Freitas AR, Silva TSO, Ribeiro RF, et al. Oral bacterial colonization on dental implants restored with titanium or zirconia abutments: 6-month follow-up. Clin Oral Investig. 2018;22(6):2335-2343.
10. Galindo-Moreno P, León-Cano A, Ortega-Oller I, et al. Prosthetic abutment height is a key factor in peri-implant marginal bone loss. J Dent Res. 2014;93(7 suppl):80S-85S.
11. Canullo L, Peñarrocha-Oltra D, Covani U, Rossetti PH. Microbiologic and clinical findings of implants in healthy condition and with peri-implantitis. Int J Oral Maxillofac Implants. 2015;30(4):834-842.
12. Lang NP, Berglundh T; Working Group 4 of Seventh European Workshop on Periodontology. Periimplant diseases: where are we now? - Consensus of the Seventh European Workshop on Periodontology. J Clin Periodontol. 2011;38 suppl 11:178-181.
13. Happe A, Röling N, Schäfer A, Rothamel D. Effects of different polishing protocols on the surface roughness of Y-TZP surfaces used for custom-made implant abutments: a controlled morphologic SEM and profilometric pilot study. J Prosthet Dent. 2015;113(5):440-447.
14. Shapoff CA, Babushkin JA, Wohl DJ. Clinical use of laser-microtextured abutments: a case series. Int J Periodontics Restorative Dent. 2016;39(5):655-662.
15. Blázquez-Hinarejos M, Ayuso-Montero R, Álvarez-López JM, et al. Histological differences in the adherence of connective tissue to laser-treated abutments and standard abutments for dental implants. An experimental pilot study in humans. Med Oral Patol Oral Cir Bucal.2017;22(6):e774-e779.
16. Mombelli A, Décaillet F. The characteristics of biofilms in peri-implant disease. J Clin Periodontol.2011;38 suppl 11:203-213.
17.de Avila ED, Vergani CE, Mollo Junior FA, et al. Effect of titanium and zirconia dental implant abutments on a cultivable polymicrobial saliva community. J Prosthet Dent.2017;118(4):481-487.
18. Nascimento Cd, Pita MS, Santos Ede S, et al. Microbiome of titanium and zirconia dental implants abutments. Dent Mater.2016;32(1):93-101.
19. Linkevicius T, Vaitelis J. The effect of zirconia or titanium as abutment material on soft peri-implant tissues: a systematic review and meta-analysis. Clin Oral Implants Res.2015;26 suppl 11:139-147.
20. Blanco J, Pico A, Caneiro L, et al. Effect of abutment height on interproximal implant bone level in the early healing: a randomized clinical trial. Clin Oral Implants Res.2018;29(1):108-117.
21.Pico A, Martín-Lancharro P, Caneiro L, et al. Influence of abutment height and implant depth position on interproximal peri-implant bonein sites with thin mucosa. A 1-year randomized clinical trial. Clin Oral Implants Res.2019;30(7):595-602.
22. Spinato S, Galindo-Moreno P, Bernardello F, Zaffe D. Minimum abutment height to eliminate bone loss: influence of implant neck design and platform switching. Int J Oral Maxillofac Implants.2018;33
(2):405-411.
23. Mishra SK, Chowdhary R, Kumari S. Microleakage at the different implant abutment interface: a systematic review. J Clin Diagn Res.2017;11(6):ZE10-ZE15.
24. Black DL, Turkyilmaz I, Lien W, Chong CH. Evaluation of the sealing capability of the internal conical connections of implants with titanium and zirconia abutments. J Contemp Dent Pract.2017;18(10):915-922.
25. Yuodelis R, Weaver JD, Sapkos S. Facial and lingual contours of artificial complete crown restorations and their effects on the periodontium. J Prosthet Dent. 1973;29(1):61-66.
26. Becker CM, Kaldahl WB. Current theories of crown contour, margin placement, and pontic design. J Prosthet Dent. 1981;45(3):286-277.
27. Chaves ES, Lovell JS, Tahmasebi S. Implant-supported crown design and the risk for peri-implantitis. Clin Adv Periodontics. 2014;4(2):118-126.
28. Katafuchi M, Weinstein BF, Leroux BG, et al. Restoration contour is a risk indicator for peri-implantitis: a cross-sectional radiographic analysis. J Clin Periodontol. 2018;45(2):225-232.
29. Linkevicius T, Puisys A, Vindasiute E, et al. Does residual cement around implant-supported restorations cause peri-implant disease? A retrospective case analysis. Clin Oral Implants Res. 2013;24(11):1179-1184.
30. Wilson TG Jr. The positive relationship between excess cement and peri-implant disease: a prospective clinical endoscopic study. J Periodontol. 2009;80(9):1388-1392.
31. Linkevicius T, Vindasiute E, Puisys A, Peciuliene V. The influence of margin location on the amount of undetected cement excess after delivery of cement-retained implant restorations. Clin Oral Implants Res. 2011;22(12):1379-1384.
32. Linkevicius T, Vindasiute E, Puisys A, et al. The influence of the cementation margin position on the amount of undetected cement. A prospective clinical study. Clin Oral Implants Res. 2013;24(1):71-76.
33. Vindasiute E, Puisys A, Maslova N, et al. Clinical factors influencing removal of the cement excess in implant-supported restorations. Clin Implant Dent Relat Res. 2015;17(4):771-778.
34. Staubli N, Walter C, Schmidt JC. Excess cement and the risk of peri-implant disease - a systematic review. Clin Oral Implants Res.2017;28 (10):1278-1290.
35. Serino G, Ström C. Peri-implantitis in partially edentulous patients: association with inadequate plaque control. Clin Oral Implants Res. 2009;20(2):169-174.
36. Heitz-Mayfield LJ, Salvi GE, Boticelli D, et al. Anti-infective treatment of peri-implant mucositis: a randomised controlled clinical trial. Clin Oral Implants Res. 2011:22(3):237-241.
37. Kotsakis GA, Lian Q, Ioannou AL, et al. A network meta-analysis of interproximal oral hygiene methods in the reduction of clinical indices of inflammation. J Periodontol. 2018;89(5):558-570.