You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
Dental caries remains a common public health issue. The Global Burden of Disease 2010 Study showed that untreated caries in permanent teeth was the most prevalent condition among all diseases evaluated.1 In addition, untreated caries in deciduous teeth was the tenth most prevalent condition, affecting 9% of the population worldwide.1 Similarly, the 2010 Canadian Health Measures Survey reported that 57% of children aged 6 to 11 years have experienced at least one cavity.2
It is evident that the pediatric population continues to be in need of comprehensive dental treatment. For practitioners to be able to provide safe and effective dental care to children, they must possess a fundamental understanding of pharmacological principles and how these principles differ in pediatric patients compared to adult patients. In this article, the pharmacokinetic and pharmacodynamic changes in children are discussed, with specific emphasis on the most commonly prescribed medications: local anesthetics, analgesics, and antibiotics.
Pharmacokinetics
The term pharmacokinetics refers to what an organism does to a drug after its administration, specifically, its absorption, distribution, biotransformation, and elimination.3 Various biological and physiological differences exist between children and adults; this fact ultimately affects the pharmacokinetic parameters and alters the disposition of drugs administered to pediatric patients.
Absorption
Drug absorption describes the process by which the drug is transferred from the site of administration to the blood stream. The gastric pH in children is more acidic compared to adults, resulting in an increase in absorption of acid labile drugs, such as penicillin and amoxicillin, and an associated increase in the drug's effect.4 Furthermore, prolonged gastric emptying in children may result in a delayed time to reach peak concentrations, meaning the expected actions of the drug can take longer to achieve.5 Another factor affecting absorption is intestinal transit time, which appears to be shorter in children aged 8 and younger, thereby reducing the amount of total drug absorbed.6
Distribution
Once a drug is absorbed into the blood stream, it is distributed to the body's various tissues and organs. Physiological factors influencing distribution include membrane permeability, body composition, and plasma protein binding. In terms of membrane permeability, the blood-brain barrier is less mature in children, allowing for the possible penetration of drugs and resultant associated toxicity.4 Moreover, the total body composition is significantly different in children when compared to adults. Children have much higher proportions of total body water, leading to a relatively higher volume of distribution of hydrophilic drugs and a correspondent lower plasma concentration.5
Lastly, plasma protein binding can greatly affect the distribution of drugs and the unbound fraction or free drug. Generally, basic drugs bind to globulin and α1-acid glycoprotein, while acidic drugs bind to albumin.7 In children, protein binding is usually reduced due to a decrease in the concentration of these proteins and their lower binding capacities.8,9 Also, the affinity of many drugs for albumin appears to be lower in children when compared to adults.10,11 Overall, these alterations in plasma protein binding properties lead to a higher fraction of unbound drug, which will result in a higher volume of distribution, thereby freeing up more of the drug to spread to the rest of the body and intensifying its pharmacological effects.
Biotransformation
The primary objective of biotransformation is to change drugs into more water-soluble entities to aid in their elimination. The main organ involved in biotransformation is the liver, which constitutes 5% of body weight at birth but only 2% in adults.12 Most drugs are metabolized in two phases. Phase I reactions involve structural alterations to the molecule, while phase II reactions consist of conjugation with another more water-soluble moiety to enhance excretion.4 Both phase I and phase II metabolic pathways are immature in children, leading to differences in biotransformation of drugs and decreased hepatic clearance.5,13 A reduced intrinsic metabolic activity of the liver, including the CYP 450 dependent enzyme system, can increase the amount of drug in the plasma and predispose pediatric patients to exaggerated or toxic effects. Furthermore, children have increased hepatic blood flow due to a larger liver-to-total body mass ratio, meaning the drug will be introduced to the liver at a faster rate.6
Elimination
The major organ involved in the excretion mechanism is the kidney. Kidney function is not fully developed at a young age and children experience reduced renal blood flow, glomerular filtration rates, and tubular secretion rates in comparison to adults.4,13 These factors impair the elimination of drugs and can lead to toxicity and other adverse effects if doses are not properly adjusted.
In summary, these pharmacokinetic differences in pediatric patients tend to raise the plasma concentrations of drugs, thereby increasing their risk of adverse events secondary to toxicity.
Pharmacodynamics
Pharmacodynamics describes the observed effect on the body resulting from a certain drug concentration.14 In children, these effects can be influenced by physiological differences or disease processes. More specifically, the number of receptors and their affinity for various drugs can change over time or be underdeveloped in children.13 In pediatric dentistry, pharmacodynamic concepts are especially important with regard to central nervous system (CNS) medications frequently prescribed during sedation.
Most pediatric patients undergoing conscious sedation during dental procedures receive nitrous oxide and oxygen in combination with a benzodiazepine, such as midazolam. Benzodiazepines act on GABAA receptors in the CNS to hyperpolarize cells and lower brain activity, thereby producing anxiolytic and amnestic effects.15 However, GABA has been shown to be the main excitatory neurotransmitter in pediatric patients.16 Consequently, paradoxical reactions characterized by agitation, disorientation, inconsolable crying, and restlessness have been reported in children receiving midazolam.15 This finding clearly illustrates an example of how pharmacodynamic changes in children as compared to adults must be taken into account to avoid significant adverse events.
Commonly Prescribed Medications in Dentistry
Prescribing medications for pediatric patients usually requires adjusting the dose to the weight of the child and altering the frequency in order to prevent serious side effects or toxicity. Commonly prescribed medications in daily dental practice include local anesthetics, antibiotics, and analgesics. These will be discussed with emphasis on required considerations and modifications for pediatric dental patients.
Local Anesthetics
The use of local anesthetics in the dental office is considered safe when they are properly administered according to guidelines. Basic pharmacological principles of local anesthetics apply to children with no significant differences expected as compared to adults. For example, just as in the case of an adult patient, the dose of epinephrine contained in local anesthetic should be revised and limited in a child with cardiovascular disease. However, pediatric patients have a higher risk of experiencing toxicity and serious adverse events due to their decreased body weight and frequent use of sedation.
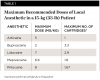
Although predisposition to toxic effects is patient-specific and depends on several factors, the primary cause of local anesthetic toxicity in children is an exaggerated dose. The maximum recommended doses for pediatric patients are summarized in Table 1.17,18 It is crucial to note the small amount of local anesthetic that should be given to a child. Furthermore, it is clear that the higher-concentration solutions, such as articaine and prilocaine, should be used with caution as toxic levels will be reached much more rapidly when compared to the other drugs available. Another factor influencing the choice of local anesthetic is the presence of a vasoconstrictor. Plain local anesthetics devoid of a vasoconstrictor will lead to faster systemic absorption, which could result in an overdose.19 In addition, postoperative soft-tissue trauma is much more likely in children when compared to adults. For this reason, bupivacaine should be avoided in pediatric patients due to its longer duration of soft-tissue anesthesia.17
Therefore, a pediatric patient in need of full-mouth restorations and/or extractions should have a treatment plan that includes multiple appointments and quadrant dentistry to avoid exceeding the recommended maximum dose of local anesthetic. Even if the patient is very cooperative and could theoretically tolerate treatment in multiple quadrants, strict adherence to local anesthetic guidelines is suggested to prevent an inadvertent overdose. Table 2 delineates example calculations of maximum doses for a pediatric patient. Based on all of the information presented, 2% lidocaine with 1:100,000 epinephrine is the ideal choice of local anesthetic for children.
As mentioned previously, the majority of younger children require some form of pharmacological behavior management so they may endure and cooperate during dental appointments. It is important to note that both local anesthetics and sedative agents depress the CNS and will have additive effects when used together. Initial signs of local anesthetic overdose include dizziness, confusion, and circumoral numbness, whereas later signs comprise slowed speech, seizures, unconsciousness, and respiratory arrest.20,21 Most overdose complications in children typically involve neurologic and respiratory events prior to cardiovascular ones, which may include hypotension, bradycardia, and cardiovascular arrest.20 The combination of local anesthetic with opioids or antihistamines may further predispose children to seizures and adverse outcomes.17 Moreover, it is common practice to consciously sedate pediatric patients with nitrous oxide and oxygen with a benzodiazepine premedication. When administered with local anesthetic, benzodiazepines increase the seizure threshold and can, therefore, mask early signs of local anesthetic overdose (neurologic and CNS symptoms) and directly result in cardiovascular collapse.20
Antibiotics
Generally, no specific modifications are required in the pharmacotherapy of antibiotics for healthy pediatric patients. The most commonly prescribed antibiotics in daily dental practice are β-lactams, including penicillin and amoxicillin. These represent acid labile drugs and will be absorbed more readily in children than in adults due to decreased gastric pH.4 Therefore, dental practitioners should adjust the dose based on the patient's weight and take into account the fact that common antibiotic side effects may be exaggerated in children.
Odontogenic infections are among the most common infections of the oral cavity.22,23 No changes in the composition of oral bacteria found in these infections have been reported for pediatric patients. They remain polymicrobial in nature with anaerobic bacteria outnumbering the aerobes, generally by a factor of three- to fourfold.24,25 β-lactam antibiotics remain the gold standard for treatment, although their efficacy has decreased due to rising rates of antibiotic resistance and β-lactamase-producing microorganism.25 The addition of a β-lactamase inhibitor such as clavulanic acid to the β-lactam antibiotic can widen the spectrum of activity and effectively kill these resistant bacteria.23 In instances of a penicillin allergy, a popular alternative is clindamycin. It remains effective against the microorganisms causing odontogenic infections, and its main adverse effect, pseudomembranous colitis, is less frequently encountered in children.26
To prevent the development of antibiotic resistance, dental clinicians must be prudent in prescribing antibiotics only when indicated. Children presenting with acute symptoms of localized infections such as a draining fistula, pulpitis, or localized intraoral swelling should not be given antibiotics. Definitive treatment (ie, pulpotomy, pulpectomy, or extraction) should be rendered instead. Also, luxation injuries in the primary dentition do not warrant antimicrobial therapy.27 With regard to pericoronitis, evidence shows that antibiotics should be prescribed only when surgical removal of the tooth is impossible (ie, in a case of significant trismus) or when systemic infection and pyrexia is present.28 On the other hand, pediatric patients presenting with facial cellulitis secondary to an odontogenic infection and systemic involvement (ie, fever, lymphadenopathy, and/or swelling) should be treated with intravenous antibiotics as quickly as possible.27 In many cases, this requires referral to a hospital and appropriate follow-up to extract the offending tooth when the swelling is controlled. The pediatric doses for commonly prescribed antibiotics in the dental setting are presented in Table 3.29
Analgesics
The management of acute postoperative pain in children remains a fundamental priority in dental practice. The most commonly prescribed analgesics for dental pain in children are acetaminophen and ibuprofen. Although the risk of developing toxic reactions to and common adverse effects from these medications remains present, their incidence is exceedingly lower in children when compared to adults.30,31 Furthermore, it should be noted that aspirin (acetylsalicylic acid [ASA]) is contraindicated in pediatric patients as it can lead to Reye's syndrome.32,33
Acetaminophen is indicated for mild to moderate pain and is the analgesic of choice to manage acute dental pain in children.32 It is characterized by analgesic and antipyretic properties while avoiding the common side effects associated with nonsteroidal anti-inflammatory drugs (NSAIDs). Most reported incidences of acetaminophen toxicity in children are due to inadvertent overdoses where the child consumed a very large amount of medication.30 Thus, acetaminophen is very safe and effective if prescribed at appropriate therapeutic doses as listed in Table 4.29,32 It is critical to note that the child's parent or guardian must be instructed to follow the recommended dosing intervals, because often the child is only given a single dose postoperatively, which may not be enough to relieve the pain.
In most pediatric dentistry cases, acetaminophen administered properly is sufficient for pain management. However, in instances of moderate to severe pain, NSAIDs or acetaminophen in combination with an opioid may be considered. NSAIDs have the added advantage of being anti-inflammatory, but they also present multiple challenges with regard to their drug interactions and adverse effects. The latter include bleeding complications, allergic reactions, and gastrointestinal tract and renal complications.33 Long-term use of NSAIDs may lead to nephrotoxicity and should be avoided.32 The pediatric doses of ibuprofen and naproxen are detailed in Table 4.29,32
Opioids should rarely be used for the management of acute severe postoperative dental pain. If chosen, it is highly recommended that the non-opioid (acetaminophen or an NSAID) be maximized before an opioid is added.32 The appropriate pediatric dose for codeine in combination with a non-opioid is listed in Table 4.29,32 Opioids produce several side effects, including nausea, vomiting, constipation, respiratory depression, and sedation.34 Of particular importance to pediatric dentistry is the concern regarding the concurrent use of an opioid with other CNS depressants such as benzodiazepines or other sedatives, which can produce unwanted additive effects of sedation.3
Conclusion
In order to provide safe and effective dental treatment for children, pharmacotherapy might be required. Dental practitioners must be aware of the various pharmacokinetic and pharmacodynamic differences associated with pediatric patients, and not simply treat them as "tiny adults." When used, commonly prescribed medications (ie, local anesthetics, antibiotics, and analgesics) must be dose-adjusted appropriately to avoid significant adverse events and toxicity in children. Furthermore, dentists should be aware of the indications for the use of each medication prescribed and only initiate pharmacotherapy when absolutely needed.
About the Authors
William Nicola, DDS
Pediatric Resident, Faculty of Dentistry, University of Toronto, Toronto, Ontario, Canada
Aviv Ouanounou, DDS, MSc
Assistant Professor, Department of Clinical Sciences (Pharmacology and Preventive Dentistry), Faculty of Dentistry, University of Toronto, Toronto, Ontario, Canada; Private Practice, Toronto, Ontario, Canada
Queries to the author regarding this course may be submitted to authorqueries@aegiscomm.com.
References
1. Marcenes W, Kassebaum NJ, Bernabé E, et al. Global burden of oral conditions in 1990-2010: a systematic analysis. J Dent Res. 2013;92(7):592-597.
2. Health Canada. Report on the findings of the oral health component of the Canadian Health Measures Survey 2007-2009. Ministry of Health, Government of Canada. 2010. http://publications.gc.ca/site/eng/369649/publication.html. Accessed April 24, 2019.
3. Ouanounou A, Haas DA. Pharmacotherapy for the elderly dental patient. J Can Dent Assoc. 2015;80:f18.
4. Fernandez E, Perez R, Hernandez A, et al. Factors and mechanisms for pharmacokinetic differences between pediatric population and adults. Pharmaceutics. 2011;3(1):53-72.
5. Strolin Benedetti M, Baltes EL. Drug metabolism and disposition in children. Fundam Clin Pharmacol. 2003;17(3):281-299.
6. Batchelor HK, Marriott JF. Paediatric pharmacokinetics: key considerations. Br J Clin Pharmacol. 2015;79(3):395-404.
7. Strolin Benedetti M, Whomsley R, Baltes EL. Differences in absorption, distribution, metabolism and excretion of xenobiotics between the paediatric and adult populations. Expert Opin Drug Metab Toxicol. 2005;1(3):447-471.
8. Kearns GL, Abdel-Rahman SM, Alander SW, et al. Developmental pharmacology-drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349(12):1157-1167.
9. Kurz H, Michels H, Stickel HH. Differences in the binding of drugs to plasma proteins from newborn and adult man. II. Eur J Clin Pharmacol. 1977;11(6):469-472.
10. Wallace S. Altered plasma albumin in the newborn infant. Br J Clin Pharmacol. 1977;4(1):82-85.
11. Morselli PL, Franco-Morselli R, Bossi L. Clinical pharmacokinetics in newborns and infants. Age-related differences and therapeutic implications. Clin Pharmacokinet. 1980;5(6):485-527.
12. Benedetti MS, Whomsley R, Canning M. Drug metabolism in the paediatric population and in the elderly. Drug Discov Today. 2007;12(15-16):599-610.
13. Lu H, Rosenbaum S. Developmental pharmacokinetics in pediatric populations. J Pediatr Pharmacol Ther. 2014;19(4):262-276.
14. Meibohm B, Derendorf H. Basic concepts of pharmacokinetic/pharmacodynamic (PK/PD) modelling. Int J Clin Pharmacol Ther. 1997;35(10):401-413.
15. Mahajan C, Dash HH. Procedural sedation and analgesia in pediatric patients. J Pediatr Neurosci. 2014;9(1):1-6.
16. Leinekugel X, Khalilov I, McLean H, et al. GABA is the principal fast-acting excitatory transmitter in the neonatal brain. Adv Neurol. 1999;79:189-201.
17. Haas DA. An update on local anesthetics in dentistry. J Can Dent Assoc. 2002;68(9):546-551.
18. Yagiela JA. Local anesthetics. In: Dionne RA, Phero JC, Becker DE, eds. Pain and Anxiety Control in Dentistry. Philadelphia, PA: W.B. Saunders; 2002:78-96.
19. Becker DE, Reed KL. Essentials of local anesthetic pharmacology. Anesth Prog. 2006;53(3):98-108.
20. Sekimoto K, Tobe M, Saito S. Local anesthetic toxicity: acute and chronic management. Acute Med Surg. 2017;4(2):152-160.
21. Moore PA, Hersh EV. Local anesthetics: pharmacology and toxicity. Dent Clin North Am. 2010;54(4):587-599.
22. Bali RK, Sharma P, Gaba S, et al. A review of complications of odontogenic infections. Natl J Maxillofac Surg. 2015;6(2):136-143.
23. Sandor GK, Low DE, Judd PL, Davidson RJ. Antimicrobial treatment options in the management of odontogenic infections. J Can Dent Assoc. 1998;64(7):508-514.
24. Baker KA, Fotos PG. The management of odontogenic infections. A rationale for appropriate chemotherapy. Dent Clin North Am. 1994;38(4):689-706.
25. Heimdahl A, von Konow L, Nord CE. Isolation of beta-lactamase-producing Bacteroides strains associated with clinical failures with penicillin treatment of human orofacial infections. Arch Oral Biol. 1980;25(10):689-692.
26. Brook I. Pseudomembranous colitis in children. J Gastroenterol Hepatol. 2005;20(2):182-186.
27. American Academy of Pediatric Dentistry. Use of Antibiotic Therapy for Pediatric Dental Patients. 2014. https://www.aapd.org/globalassets/media/policies_guidelines/bp_antibiotictherapy.pdf. Accessed April 24, 2019.
28. Renton T, Wilson NH. Problems with erupting wisdom teeth: signs, symptoms, and management. Br J Gen Pract. 2016;66(649):e606-e608.
29. American Academy of Pediatric Dentistry. Useful Medications for Oral Conditions. http://www.aapd.org/media/policies_guidelines/rs_commonmeds.pdf. Accessed April 24, 2019.
30. American Academy of Pediatrics. Committee on Drugs. Acetaminophen toxicity in children. Pediatrics. 2001;108(4):1020-1024.
31. Lesko SM, Mitchell AA. The safety of acetaminophen and ibuprofen among children younger than two years old. Pediatrics. 1999;104(4):e39.
32. Haas DA. An update on analgesics for the management of acute postoperative dental pain. J Can Dent Assoc. 2002;68(8):476-482.
33. Ong KS, Seymour RA. Maximizing the safety of nonsteroidal anti-inflammatory drug use for postoperative dental pain: an evidence-based approach. Anesth Prog. 2003;50(2):62-74.
34. Jitpakdee T, Mandee S. Strategies for preventing side effects of systemic opioid in postoperative pediatric patients. Paediatr Anaesth. 2014;24(6):561-568.