You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
The importance of diagnosis, risk assessment, treatment planning, and prevention of oral diseases for older adults cannot be overstated.1-3 While a healthy mouth and beautiful smile are often symbolic of youthfulness and good overall health, tooth loss is associated with aging and pathologic changes in the oral cavity that can portend the presence of systemic diseases. The etiology and risk factors for caries, periodontal disease, oral pathology, and tooth loss as they relate to systemic diseases must be considered. Because older adults are managing chronic diseases over a span of decades and the severity and associated acute conditions are manifested differently among individuals and populations, a “one-size-fits-all” approach to providing oral healthcare is inadequate.2
The Seattle Care Pathway (SCP) offers practitioners a systematic way to assess the risks of functional dependence—whether the dependence is due to disease or is a result of normal aging processes in advanced age—on oral health and the provision of care. SCP includes assessment of risks to oral health, preventive options, treatment considerations, and guidelines for collaboration and communications to improve outcomes in care.2 Older adults without systemic diseases have all the treatment options available to them as younger adults have. Similarly, those with well-controlled systemic disease(s), considered pre-dependent per SCP, also have broad treatment options but must be aware of the potential risks their systemic disease(s) carries for their oral health. Those with moderate dependency whose diseases are not well controlled and impact oral health will require more interaction among healthcare providers, additional prevention, modifications in treatment planning, and increased interprofessional communications. For older adults with high dependency, a coordinated interdisciplinary team effort is critical to identify and limit the risks to their oral health and to provide safe, appropriate care. Treatment options for these patients may be limited to prevention or palliation. Thus, intervention with information and resources for well and pre-dependent older adults is vital to prevent the onset of oral diseases that can become more difficult to manage as comorbidities increase and/or the patients become more dependent.2
Oral Cancer
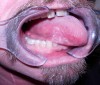
Oral and oral pharyngeal cancer is perhaps the clearest example of an oral disease as systemic disease (Figure 1). Due to having a longer history of exposure to carcinogens, such as tobacco and alcohol, older adults may be more disposed to oral cancers compared to younger populations. Plus, more recently, human papillomavirus has been found to be a significant risk factor for oropharyngeal cancers in younger populations with a potential to adversely impact oral health and function in future generations of older adults.4
In 2016 oral cancer was estimated to account for 2.5% of all cancers.5 The prevalence of oral cancer has declined but oropharyngeal cancers have increased.4 The 5-year survival rate for oropharyngeal cancers is estimated at only 64%, but survival rates can be improved with earlier detection.5 Oral cancer can metastasize to other sites or occur as a metastasis from another site, such as from breast cancer.4,6 Providing minimally invasive screening for oral cancers on a regular basis in all older adults is a key to intervening at the earliest stages and minimizing the potential for extensive surgery and/or radiation or chemotherapy.
The oral mucosa and salivary system are vulnerable to the effects of chemotherapy and radiation, and surgery can result in disfiguration, intense oral pain, and dysfunction. These effects can be so severe that therapy may be interrupted to allow patients to maintain basic nutrition.7,8 Even if treatment and cure is not the goal for elderly patients too frail to tolerate treatment, identification of oral cancer can allow health practitioners to monitor and possibly limit the advancement of the disease and provide palliative care to maintain comfort, nutrition, and quality of life.2,9
During cancer treatment, dental care may be limited to palliation of side effects and/or aggressive prevention of oral diseases that could occur in conjunction with treatment (Table 1).10-12 One example is xerostomia, which can increase the risk of caries and cause significant discomfort and problems with taste, swallowing, and speech. Another is painful mucositis that interferes with or prevents routine oral hygiene. Some patients may require special diets that could heighten the risk of caries due to increased frequency of meals or soft, moist food textures that may be difficult to remove from the tooth surfaces. Patients may be immunosuppressed and at risk for opportunistic infections or increased bleeding. Practitioners should carefully review the treatment regimens and chemotherapeutics either currently in use or previously used to determine if they have the potential for such side effects. Laboratory tests may be needed to assess the patient risk of adverse treatment outcomes. For example, neutrophil counts should be above 2 k/mm and platelets above 60 k to consider proceeding with routine care.8 If emergency dental care is needed and palliative care will not suffice, patients can be referred to a hospital. Patients who have successfully completed care will require routine follow-up for recurrent cancers. Furthermore, if patients have received either radiation to the head and neck area (greater than 5,500 cGy), such as for treatment of oral cancer,7,13 or intravenous bisphosphonate therapy for cancers, such as for metastatic bone cancer, they can be at risk for osteonecrosis. Although the etiologies, prevention, and management are distinct in each case, poorly fitting prostheses, untreated oral diseases, and invasive bony procedures are risk factors for the development of osteonecrosis in both of these groups of patients. Their risk for osteonecrosis must be assessed routinely and considered in treatment planning and management.7,14
Xerostomia and Salivary Hypofunction
Saliva serves many important functions. These include pH maintenance, helping enable taste, immune function, tooth remineralization, and playing a role in the beginning of digestion.15 Hyposalivation and sensations of dry mouth (xerostomia) are often experienced by older adults and related to systemic disease. There are many classes of medications (Table 2 through Table 5) commonly used by older adults that cause hyposalivation and/or xerostomia.2,7,10,13,16-22 (Note: Table 2 through Table 5 are examples of common issues related to oral healthcare and are not intended to provide a comprehensive list.)
In cases where only a single xerostomic medication is prescribed, an inquiry to the physician about prescribing a medication with less severe impact on the salivary system could be indicated; however, for patients with polypharmacy, several medications may be responsible for the xerostomia, making it unlikely the physician can alter the regimen successfully. In addition, if there is no drug available that can be used to achieve the same therapeutic result then palliative and preventive management of dry mouth will be required.16
Diseases such as Sjogren’s syndrome, hepatitis C, and Parkinson’s disease can also alter salivary function.23 Individuals lacking adequate saliva can have difficulty wearing dentures due to poor retention or discomfort and trauma of the dry and fragile tissues. One age-related change in the oral cavity is thinning mucosa, which can become more susceptible to trauma. Dryness of the tissues increases the risk of trauma.16,23,24 Individuals with severe salivary hypofunction may experience difficulty swallowing and even speaking.
Opportunistic fungal infections also can occur in such an environment, particularly in patients with uncontrolled systemic diseases such as diabetes, malnutrition, immunosuppression, and immunocompromise.23 Gustatory sensation is diminished in the absence of saliva, which can then result in limited or altered dietary choices, sometimes leading to poor intake and/or malnutrition.16 For adults who already have limited diets due to systemic diseases, these additional compromises in quality of life and enjoyment of social activities and food can have a profound and negative impact on health and well-being.10,16,18 Psychosocial complications such as frustration, embarrassment, and unhappiness also have been reported to result from these sequelae and are of particular concern in older adults who may already be at risk for social isolation and/or are suffering from depression.25
Salivary stimulants, such as pilocarpine, may be used for individuals with severe dry mouth as a result of head and neck radiation or Sjogren’s syndrome. For dry mouth resulting from other causes, topical rinses, gels, and sprays can provide relief. Many such products are available and the decision of which one(s) to use, along with how and when to use it, will depend on the desired effect.15,24 Some may also be used in combination with other aids such as fluorides or products that neutralize the pH to prevent caries.19
Dental Caries
Caries are one of the most serious risks to oral health. Evidence suggests that the burden of untreated caries is shifting toward adulthood with peak prevalence among adults at age 70.26 Among community-dwelling adults in the United States aged 65 and older 19% have untreated coronal caries, while the prevalence of root caries is 14%.27
CAMBRA (caries management by risk assessment) is an evidence-based approach that uses each patient’s unique caries risk profile (pH, salivary flow, lifestyle habits, etc) to prevent, reverse, and/or repair damage to teeth. This is done through the prescription of appropriate chemotherapeutics for home use, the application of in-office fluoride, and the use of minimally invasive procedures such as placement of silver diamine fluoride to arrest caries and glass-ionomer restorations to prevent disease and preserve tooth structure.28,29 According to CAMBRA criteria, salivary hypofunction alone places individuals at extreme caries risk, which can be further compounded by additional risks such as an acidic oral environment, poor oral hygiene, and a history of caries experience. Evidence supports less invasive carious lesion management as opposed to traditional methods that focus on placing restorations.30 Partial caries removal31 and sometimes no caries removal may be indicated.32 This can be especially beneficial for frail patients who are unable to tolerate or cooperate with extensive procedures or unable to seek care in traditional dental practices.2
Additional comorbidities and increasing dependence on others also escalates caries risk.2,33 Treatment planning with these limitations and risks in mind is critical to ensure an appropriate plan is proposed to the patient. For example, individuals who are left with residual functional impairment following a stroke may be challenged to perform their own oral hygiene. Some patients will require assistance from caregivers to adequately address their oral hygiene needs for natural teeth as well as prostheses. Lack of motivation and understanding, dietary restrictions, or behavior that prevents oral care also contribute to caries risk.34 Patients with oral motor dysfunction resulting in chewing and swallowing problems following stroke, or as a result of progressive neurological diseases such as Parkinson’s disease or multiple sclerosis, may need to alter their diet and eat foods that are soft or pureed or require thickened liquids. Regular intake of foods of these textures and/or poor food clearance can pose a caries risk to these patients if their oral hygiene is inadequate and additional chemotherapeutic preventive measures are not utilized.34
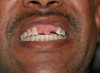
Rapidly advancing and rampant caries can result in pain, acute and chronic infections, and tooth loss leading to resultant functional, esthetic, and psychological sequelae of poor oral health.25 Left untreated, root caries can result in the loss of the crown and a retained root that can be a chronic source of inflammation (Figure 2). This, in turn, causes exposure of the pulp chamber, creating a risk for chronic and acute apical periodontitis.20
Acute exacerbation of a chronic abscess can lead to serious complications, especially for patients who are already medically compromised.20 In addition to severe pain, infection that spreads through facial planes can result in substantial costs for care annually to treat life-threatening conditions such as Ludwig’s angina.35
Periodontal Diseases
Periodontal disease is a chronic inflammatory disease affecting 70% of adults aged 65 and older; of those, 11% have severe periodontal disease resulting in tooth loss.36 Severe chronic periodontitis is the sixth most prevalent condition affecting the global population37 with a 7.6% prevalence rate and an incidence of 6 million cases worldwide in the past 25 years.38 Periodontal disease has been linked to multiple systemic conditions, including diabetes, cardiovascular disease, and respiratory diseases.20 Indeed, the Santa Fe Group has recently concluded that periodontal disease is a “contributory cause” to certain systemic diseases, and the public should benefit from this knowledge.39 Moderate to severe chronic periodontitis is characterized by tissue destruction. Bacteria and the byproducts of inflammation constantly enter the blood stream through the ulcerated periodontal tissues, generating an adaptive immune response that contributes to the development of systemic conditions. Three common mechanisms form the periodontal-systemic feedback loop: tissue homeostasis, immune cell phenotype, and serum lipid levels.40
Periodontitis is a risk factor for diabetes and atherosclerotic cerebrovascular disease (ACVD). As interdependent diseases that share pathological pathways, treatment of one condition may affect the other. Some studies have shown statins prevent and reverse both ACVD and periodontitis, while some studies have demonstrated that treatment of periodontal disease improves glycemic control and reduces HbA1C levels.41,42
Systemic diseases can directly impact the periodontium, such as with poorly controlled diabetes, or they can indirectly increase the risk of periodontal disease due to diminishing ability to provide self-care.2,25,34 Communicating these risks to patients themselves, caregivers, and other healthcare professionals is vital in preventing onset and progression of periodontal diseases that are associated with systemic diseases. Patients may also benefit from increased recall visits and modifications in their oral hygiene routine as their physical and cognitive abilities are altered throughout the course of their systemic diseases. This can occur with simple measures ranging from adaptive aids set up, to reminders to brush and floss, to full assistance twice daily by a caregiver.34
Tooth Loss
Tooth loss can have important consequences for older adults, especially those managing multiple comorbid conditions.25 Some loss of chewing proficiency comes with aging. This can become significant when it is compounded by discomfort caused by periodontal disease and dental caries that result in tooth loss that further alters masticatory efficiency. Diet and nutrition can be significantly impacted, especially when fewer than 20 natural teeth (10 occluding pairs) remain. Inefficient mastication can result in digestive problems, poor food choices, nutritional deficiencies, and diminished quality of life.43 Prosthetic rehabilitation of posterior teeth can improve chewing efficiency and reduce the risk of malnutrition43; however, the decision of whether or not to restore missing teeth is often more complicated than anticipated.
Some individuals may have significant oral motor dysfunction, such as those who have had a stroke resulting in dysphagia or dysphasia or have limited ability to maintain their oral hygiene due to physical or cognitive impairments such as individuals with Alzheimer’s or Parkinson’s disease. Patients with limited oral motor function may be unsuccessful in coordinating their oral musculature to use removable prostheses. Those with dexterity or cognitive problems may be unable to maintain the oral hygiene necessary for fixed prostheses. Sometimes significant pathology arises from poorly fitting or failing existing prostheses and pre-prosthetic surgery must be performed to make a new prosthesis (Figure 3). For patients with poorly controlled systemic disease, such as diabetes, or those at significant risk for an adverse outcome such as individuals with advanced and poorly controlled cardiac diseases, the high risk of poor outcomes may prevent invasive procedures needed to enable replacement of missing teeth. The best treatment option in some cases may be to not replace missing teeth, but instead focus on maintaining the health of a reduced but functional dentition.2
Conclusion
Teeth are an important part of individual identity. Poor oral health can have a negative effect on interpersonal relationships. Dental pain can affect focus, work, and sleep. Tooth loss is associated with lower confidence, altered self-image, isolation, depression, and diminished quality of life.25,44 Older adults are a heterogeneous group in regard to overall health, oral health, socioeconomic status, and health literacy, but issues of comfort, identity, and dignity are universal.44 To age successfully and with dignity, seniors must have the opportunity to benefit from oral healthcare as a component of chronic disease management and as an integral element of healthcare.
About the Authors
Elisa M. Chávez, DDS
Associate Professor
Department of Diagnostic Sciences
University of the Pacific
Dugoni School of Dentistry
San Francisco, California
Amruta Hendre, DDS
Adjunct Clinical Faculty
University of the Pacific
Dugoni School of Dentistry
San Francisco, California
Dentist
On Lok Lifeways
San Francisco, California
Queries to the author regarding this course may be submitted to authorqueries@aegiscomm.com.
References
1. Shay K. Identifying the needs of the elderly dental patient. The geriatric dental assessment. Dent Clin North Am. 1994;38(3):499-523.
2. Pretty IA, Ellwood RP, Lo EC, et al. The Seattle Care Pathway for securing oral health in older patients. Gerodontology. 2014;31 suppl 1:77-87.
3. Oong EM, An GK. Treatment planning considerations in older adults. Dent Clin North Am. 2014;58(4):739-755.
4. Weatherspoon DJ, Chattopadhyay A, Boroumand S, Garcia I. Oral cavity and oropharyngeal cancer incidence trends and disparities in the United States: 2000-2010. Cancer Epidemiol. 2015;39(4):497-504.
5. Cancer Statistics, Statistical Summaries, Cancer Stat Facts: Oral Cavity and Pharynx Cancer. National Cancer Institute website. https://seer.cancer.gov/statfacts/html/oralcav.html. 2017. Accessed June 20, 2017.
6. Noguti J, De Moura CF, De Jesus GP, et al. Metastasis from oral cancer: an overview. Cancer Genomics Proteomics. 2012;9(5):329-335.
7. Jawad H, Hodson NA, Nixon PJ. A review of dental treatment of head and neck cancer patients, before, during and after radiotherapy: Part 1. Br Dent J. 2015;218(2):65-68.
8. Chung EM, Sung EC. Dental management of chemoradiation patients. J Calif Dent Assoc. 2006;34(9):735-742.
9. Shay K. Dental management considerations for institutionalized geriatric patients. J Prosthet Dent. 1994;72(5):510-516.
10. Singh ML, Papas A. Oral implications of polypharmacy in the elderly. Dent Clin North Am. 2014;58(4):783-796.
11. Yuan A, Woo S-B. Adverse drug events in the oral cavity. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;119(1):35-47.
12. Jacobsen PL, Chávez EM. Clinical management of the dental patient taking multiple drugs. J Contemp Dent Pract. 2005;6(4):144-151.
13. Chrcanovic BR, Reher P, Sousa AA, Harris M. Osteoradionecrosis of the jaws—a current overview—Part 2: dental management and therapeutic options for treatment. Oral Maxillofac Surg. 2010;14(2):81-95.
14. Vescovi P, Campisi G, Fusco V, et al. Surgery-triggered and non surgery-triggered bisphosphonate-related osteonecrosis of the jaws (BRONJ): a retrospective analysis of 567 cases in an Italian multicenter study. Oral Oncol. 2011;47(3):191-194.
15. Epstein JB, Beier Jensen S. Management of hyposalivation and xerostomia: criteria for treatment strategies. Compend Contin Educ Dent. 2015;36(8):600-603.
16. Han P, Suarez-Durall P, Mulligan R. Dry mouth: a critical topic for older adult patients. J Prosthodont Res. 2015;59(1):6-19.
17. Lovelace TL, Fox NF, Sood AJ, et al. Management of radiotherapy-induced salivary hypofunction and consequent xerostomia in patients with oral or head and neck cancer: meta-analysis and literature review. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117(5):595-607.
18. Turner MD. Hyposalivation and xerostomia: etiology, complications, and medical management. Dent Clin North Am. 2016;60(2):435-443.
19. Hurlbutt M, Young DA. A best practices approach to caries management. J Evid Based Dent Pract. 2014;14 suppl:77-86.
20. Maret D, Peters OA, Vigarios E, et al. Dental screening of medical patients for oral infections and inflammation: consideration of risk and benefit. Microbes Infect. 2017;19(2):84-90.
21. Lee KH, Wu B, Plassman BL. Cognitive function and oral health-related quality of life in older adults. J Am Geriatr Soc. 2013;61(9):1602-1607.
22. Ghezzi EM, Ship JA. Systemic diseases and their treatments in the elderly: impact on oral health. J Public Health Dent. 2000;60(4):289-296.
23. Saleh J, Figueiredo MA, Cherubini K, Salum FG. Salivary hypofunction: an update on aetiology, diagnosis and therapeutics. Arch Oral Biol. 2015;60(2):242-255.
24. Villa A, Connell CL, Abati S. Diagnosis and management of xerostomia and hyposalivation. Ther Clin Risk Manag. 2014;11:45-51.
25. Gil-Montoya JA, de Mello AL, Barrios R, et al. Oral health in the elderly patient and its impact on general well-being: a nonsystematic review. Clin Interv Aging. 2015;10:461-467.
26. Dye BA, Thornton-Evans G, Li X, Iafolla TJ. Dental caries and tooth loss in adults in the United States, 2011-2012. NCHS Data Brief. 2015;(197):197.
27. Dye BA, Tan S, Smith V, et al. Trends in oral health status: United States, 1988-1994 and 1999-2004. Vital Health Stat 11. 2007;(248):1-92.
28. Young DA, Featherstone JD, Roth JR, et al. Caries management by risk assessment: implementation guidelines. J Calif Dent Assoc. 2007;35(11):799-805.
29. Fa BA, Horst JA, Hirsch JP, et al. Caries arrest with silver diamine fluoride. Decisions in Dentistry. 2016;2(10):48-51.
30. Featherstone JD, White JM, Hoover CI, et al. A randomized clinical trial of anticaries therapies targeted according to risk assessment (caries management by risk assessment). Caries Res. 2012;46(2):118-129.
31. Thompson V, Craig RG, Curro FA, et al. Treatment of deep carious lesions by complete excavation or partial removal: a critical review. J Am Dent Assoc. 2008;139(6):705-712.
32. Schwendicke F, Frencken JE, Bjorndal L, et al. Managing carious lesions: consensus recommendations on carious tissue removal. Adv Dent Res. 2016;28(2):58-67.
33. Petersen PE, Yamamoto T. Improving the oral health of older people: the approach of the WHO Global Oral Health Programme. Community Dent Oral Epidemiol. 2005;33(2):81-92.
34. Ajwani S, Jayanti S, Burkolter N, et al. Integrated oral health care for stroke patients—a scoping review. J Clin Nurs. 2017;26(7-8):891-901.
35. Allareddy V, Rampa S, Nalliah RP, Allareddy V. Longitudinal discharge trends and outcomes after hospitalization for mouth cellulitis and Ludwig angina. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;118(5):524-531.
36. Thornton-Evans G, Eke P, Wei L, et al. Periodontitis among adults aged ≥30 years—United States, 2009-2010. MMWR Suppl. 2013;62(3):129-135.
37. Kassebaum NJ, Bernabé E, Dahiya M, et al. Global burden of severe periodontitis in 1990-2010: a systematic review and meta-regression. J Dent Res. 2014;93(11):1045-1053.
38. Kassebaum NJ, Smith AG, Bernabé E, et al. Global, regional, and national prevalence, incidence, and disability-adjusted life years for oral conditions for 195 countries, 1990-2015: a systematic analysis for the global burden of diseases, injuries, and risk factors. J Dent Res. 2017;96(4):380-387.
39. Slavkin HC, Santa Fe Group. A national imperative: oral health services in Medicare. J Am Dent Assoc. 2017;148(5):281-283.
40. Kholy KE, Genco RJ, Van Dyke TE. Oral infections and cardiovascular disease. Trends Endocrinol Metab. 2015;26(6):315-321.
41. Chapple IL, Genco R; Working group 2 of the joint EFP/AAP workshop. Diabetes and periodontal diseases: consensus report of the joint EFP/AAP workshop on periodontitis and systemic diseases. J Clin Periodontol. 2013;40 suppl 14:S106-S112.
42. Taylor GW, Borgnakke WS. Periodontal disease: associations with diabetes, glycemic control and complications. Oral Dis. 2008;14(3): 191-203.
43. Budtz-Jørgensen E, Chung JP, Rapin CH. Nutrition and oral health. Best Pract Res Clin Gastroenterol. 2001;15(6):885-896.
44. Griffin SO, Jones JA, Brunson D, et al. Burden of oral disease among older adults and implications for public health priorities. Am J Public Health. 2012;102(3):411-418.