You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
With 12 billion dosage units dispensed annually, opioid pain relievers (OPRs) are among the most frequently prescribed medications in the United States.1 At the top of the RxList for the United States is hydrocodone. In 2013, hydrocodone plus acetaminophen, eg, Vicodin®, Lorcet®, Norco®, etc., was No. 1 on that list of most prescribed drugs2; in 2011, 136 million prescriptions were dispensed, according to IMS Health.3 Oxycodone plus acetaminophen (Percocet®) was listed at No. 35 in 2013,2 with 32.8 million prescriptions dispensed in 2011.3
Opioid Prescribing Among Dentists
Among the 12 billion dosage units of opioids dispensed annually, 1 billion to 1.5 billion doses were prescribed by US dental professionals.1 Among those prescriptions, a significant portion were written for the 3.5 million patients undergoing third-molar extraction surgeries each year, with an average patient age of 20 years.1
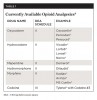
Given the potential for their misuse or abuse—especially among high-risk patients—dentists should be aware of the currently available opioid analgesics (Table 1)4 and their appropriate usage.
Opioid Indications
Opioids are most often prescribed for acute pain management in dentistry. However, they are also used as anti-tussives or anti-diarrheal agents, and sometimes as analgesics for chronic pain.
Mechanism of Action
Opioids attenuate the perception of pain by binding to opiate receptor proteins in the brain, spinal cord, and gastrointestinal tract. They can be used in combination with non-opioid analgesics, taking advantage of additive analgesia, thus blocking pain production in two distinct manners: prostaglandin inhibition by non-opioid entities, and opiate receptor activation by the opioid entity.
Common Side Effects
Common side effects of opioid use include nausea, constipation, and drowsiness. More serious side effects when taken in high doses include respiratory and central nervous system depression. Using opioids in low dosages, particularly in combination with non-opioid analgesics, can mitigate side effects.5
Practitioners should be aware that in ambulatory patients, the side effects of opioids, particularly nausea and drowsiness, may be exaggerated after office procedures to the extent that they may outweigh the pain control benefits.5
Precautions and Contraindications for Opioid Prescribing
Clinicians should also be aware of numerous contraindications for prescribing opioids, including allergy to opioids and conditions such as impaired respiratory function, paralytic ileus, history of renal disease, and history of hepatic disease.6,7
Their co-administration with drugs such as monoamine oxidase inhibitors or central nervous system depressants (eg, alcohol, benzodiazepines) can be life-threatening. Opioids are also contraindicated for both very old and very young patients, and whenever there is suspicion of active diversion, dependence, or addiction.6,7
Public Health Issues Related to Opioids
As frequent prescribers of analgesic medications, it is critical that dentists understand their potential for misuse and abuse. For example, in 2012, 1.9 million Americans used an opioid analgesic for nonmedical use for the first time.8 Moreover, 36 million Americans have used opioid analgesics for nonmedical purposes. As a consequence of the nonmedical use of opioid analgesics, there were more than 366,000 emergency department visits in 20119; in 2010, 22,134 Americans died from prescription drug overdoses, and 16,652 of those deaths involved opioid analgesics.10
Prescribing Options for Postoperative Pain
Opioids alone or in combination with acetaminophen (APAP) or ibuprofen are not the only option for treatment of postoperative dental pain. Dentists can also prescribe APAP or nonsteroidal anti-inflammatory drugs (NSAIDs) for effective management of postoperative pain. In several studies, an opioid alone produced inferior postoperative pain relief compared with NSAID or APAP alone.11-15
That said, opiates make good sense for pain that is unrelieved by other methods, eg, in patients who cannot tolerate NSAIDs but are not sufficiently relieved by the 3000 mg maximum acetaminophen dose recommended by the US Food and Drug Administration (FDA) due to concerns about liver disease at higher doses.
NSAIDs
There is a significant amount of evidence attesting to the effectiveness of NSAIDs for pain control. Studies, including those by Kleinert16 and Van Dyke,17 have found ibuprofen 400 mg to be more effective than single-entity morphine 60 mg, oxycodone 5 mg, or tapentadol at 50 mg, 75 mg, or 100 mg.16,17
It is surprising to the authors that opioids are still used so extensively and that they are offered in combination with acetaminophen, rather than the anti-inflammatory NSAIDs, which are often shown to offer better pain control with fewer side effects and can reduce swelling if continued for 2 to 3 days.
NSAIDs can be given preventively, ie, preemptively, on a fixed schedule to block the onset or lessen the severity of postoperative pain. When given either prior to surgery or just after while the surgical anesthetic is still in effect, the NSAID delays the onset of pain by more than 100 minutes18 and lessens the severity of postoperative pain.19 Pre- and postoperative administration of an NSAID was shown to achieve superior pain relief to APAP alone or in combination with oxycodone.20,21
This approach makes sense for two main reasons: it gives the drugs the opportunity to become absorbed and reach peak effectiveness by the time the anesthetic wears off; and it makes it easier to comply with FDA-recommended doses to maximize benefits and minimize risks.
Postoperative Long-Acting Local Anesthetic
Another strategy for pain control is to use the long-acting surgical anesthetic bupivacaine, which wears off slowly, rather than lidocaine, which wears off quickly, to give patients the opportunity to more gradually adjust to the pain. This is the approach now taken in the vast majority of third-molar extraction procedures. Administration of bupivacaine 0.5% with 1:200,000 epinephrine during the immediate postoperative period showed diminished pain compared to placebo and lidocaine alone at 0 to 4 hours and 48 hours.22,23
Opioids
In addition to needing relief from severe pain after surgery, dental patients often present with acute pain due to severe pulpitis or abscesses. While, as indicated above, NSAIDs and even APAP are often more effective, doctors continue to commonly prescribe opioids. This includes 85% of oral surgeons who prescribe a centrally acting opioid analgesic for postoperative pain, usually (in 64% of cases) hydrocodone/APAP.24 Among the 40.2% of analgesic prescriptions given to patients aged 18 to 30 years, 53% were for opioids.25
Combination Pain Therapy
The authors advocate using NSAIDs in combination with APAP, which have more favorable side-effect profiles than agents containing an opioid, as the primary agents for postoperative pain management, and reserving opioids for severe, continuing pain, such as might be caused by an especially traumatic procedure (Table 2).26
The authors’ recommendations conform to what is considered to be the current gold standard analgesic in moderate to severe postoperative dental pain in the United States—ibuprofen 400 mg to 600 mg and APAP 500 mg.27,28
Opioid Prescribing and Drug Abuse
According to the National Institute on Drug Abuse, the three most widely abused or misused drug classifications are opioids, central nervous system depressants, and stimulants.29 There is a frequently misleading notion in dentistry that because dentists typically prescribe opioids of limited quantity for acute pain, there is a minimal risk of diversion, dependence, or addiction. However, this could hardly be farther from the truth.4,30,31
The most commonly prescribed opioid amount is 20 doses, which is about a 3-day supply following the extraction of third molars.1 However, because patients typically use only half that amount, there is widespread—and well-founded—concern about what happens to the excess pills. Data about how pain relievers for nonmedical use are acquired32,33 indicate that most commonly, these excess pills are stored “for a rainy day” or given to friends and family (60%); if not stored securely, they may also be stolen by them (4%), or sold to a friend or relative (8%) or a drug dealer (4%).
The Role of the Dentist
Given these considerations, it is clear that dentists need to balance their responsibility to manage their patients’ dental pain against their obligation to avoid facilitating the abuse of opioid medication. There is much individual dentists can do to limit prescription drug misuse (Table 3), using numerous resources, including videos, treatment prevention literature, and assessment tools offered through the National Institute on Drug Abuse’s Medical & Health Professionals website (www.drugabuse.gov/nidamed-medical-health-professionals).31,34
They should attempt to identify potential drug abusers via any red flags raised during the intake discussion or in the patient history, and find opportunities to educate patients and parents about the health threat posed by opioid pain medications. They can do this as part of their conversation before and after procedures that require pain control.31,34
They can also re-examine their own prescribing patterns. In general, opioid prescriptions should be written with discretion to supplement the analgesic effects of NSAIDs or APAP. Refills for acute pain medication—especially those containing an opioid— should be avoided; patients who request them for legitimate reasons should return to the office and be seen, as they may be suffering from a complication that requires treatment.31,34
Practitioners should remember that pain, whether due to an abscess or surgical trauma, should quickly subside once its source is removed. Therefore, they should focus on the source, minimizing the amount of pain that is likely to result from an operative procedure by using atraumatic treatment—ie, approaches that minimize trauma to surrounding tissues, and effectively treating any possible infection.31,34
Conclusion
It is imperative that the dental community remains educated and informed of nationwide healthcare trends, and prescription drug abuse is no exception. Ethically, dentists should be able to respond in a manner that addresses the best interests of their patients.Using good clinical judgment, the dentist can fulfill his or her obligation to manage a patient’s pain, to protect the patient from unnecessary medication and abuse potential, and to maintain his or her societal responsibility to limit the diversion of opioids to the streets.
Disclosure
Dr. Moore has served as medical director and/or a research consultant to several pharmaceutical companies marketing local anesthetic products including DENTSPLY Pharmaceutical Division, Kodak Dental Systems, Septodont USA, St Renatus, Novalar, and Novocol of Canada Inc. The authors had no other disclosures to report.
About the Authors
Raymond Dionne, DDS, MS, PhD
Research Professor
Department of Pharmacology & Toxicology
Brody School of Medicine and Department of Foundational Sciences
School of Dental Medicine
East Carolina University
Greenville, North Carolina
Paul A. Moore, DMD, PhD, MPH
Professor of Pharmacology and Public Health, and former Chair of the Department of Dental Anesthesiology
University of Pittsburgh School of Dental Medicine
Pittsburgh, Pennsylvania
Queries to the author regarding this course may be submitted to authorqueries@aegiscomm.com.
References
1. Executive Summary: The Role of Dentists in Preventing Opioid Abuse. Tufts Health Care Institute Program on Opioid Risk Management. 12th Summit Meeting. March 11-12, 2010. www.thci.org/opioid/mar10docs/executivesummary.pdf. Accessed November 11, 2015.
2. Top 200 Drugs–US Only. RxList website. http://www.rxlist.com/script/main/hp.asp. Accessed November 11, 2015.
3. IMS Health. Top 25 US Pharmaceutical Products by Dispensed Prescriptions. http://www.imshealth.com/deployedfiles/ims/Global/Content/Corporate/Press%20Room/Top-Line%20Market%20Data%20%26%20Trends/2011%20Top-line%20Market%20Data/Top_Products_by_RX.pdf. Accessed November 11, 2015.
4. Oakley M, O’Donnell J, Moore PA, Martin J. The rise in prescription drug abuse: raising awareness in the dental community. Compend Contin Educ Dent. 2011;32(6):14-24.
5. National Institute on Drug Abuse. Research Report Series. Prescription Drug Abuse. http://www.drugabuse.gov/publications/research-reports/prescription-drugs/director. Accessed November 11, 2015.
6. Manchikanti L, Abdi S, Atluri S, et al. American Society of Interventional Pain Physicians (ASIPP) guidelines for responsible opioid prescribing in chronic non-cancer pain: Part 2–guidance. Pain Physician. 2012;15(3 suppl):S67-S116.
7. Hersh EV, Pinto A, Moore PA. Adverse drug interactions involving common prescription and over-the-counter analgesic agents. Clin Ther. 2007;29(suppl):2477-2497.
8. US Department of Health and Human Services. Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings. http://www.samhsa.gov/data/NSDUH/2012SummNatFindDetTables/NationalFindings/NSDUHresults2012.htm. Accessed November 11, 2015
.9. US Department of Health and Human Services. Drug Abuse Warning Network, 2011: National Estimates of Drug-Related Emergency Department Visits. http://www.samhsa.gov/data/2k13/DAWN2k11ED/DAWN2k11ED.htm. Accessed November 11, 2015.
10. Jones CM, Mack KA, Paulozzi LJ. Pharmaceutical overdose deaths, United States, 2010. JAMA. 2013;309(7):657-659.
11. Hersh EV, Moore PA, Ross GL. Over-the-counter analgesics and antipyretics: a critical assessment. Clin Ther. 2000;22(5):500-548. 12. Cooper SA. Models for clinical assessment of oral analgesics. Am J Med. 1983;75(5A):24-29.
13. Beaver WT. Aspirin and acetaminophen as constituents of analgesic combinations. Arch Intern Med. 1981;141(3 spec no):293-300.
14. Cooper SA, Precheur H, Rauch D, et al. Evaluation of oxycodone and acetaminophen in treatment of postoperative dental pain. Oral Surg Oral Med Oral Pathol. 1980;50(6):496-501.
15. Cooper SA, Engel J, Ladov M, et al. Analgesic efficacy of an ibuprofen-codeine combination. Pharmacotherapy. 1982;2(3):162-167.
16. Kleinert R, Lange C, Steup A, et al. Single dose analgesic efficacy of tapentadol in postsurgical dental pain: the results of a randomized, double-blind, placebo-controlled study. Anesth Analg. 2008;107(6):2048-2055.
17. Van Dyke T, Litkowski LJ, Kiersch TA, et al. Combination oxycodone 5 mg/ibuprofen 400 mg for the treatment of postoperative pain: a double-blind, placebo- and active-controlled parallel-group study. Clin Ther. 2004;26(12):2003-2014.
18. Dionne RA, Cooper SA. Evaluation of preoperative ibuprofen for postoperative pain after removal of third molars. Oral Surg Oral Med Oral Pathol. 1978;45(6):851-856.
19. Dionne RA, Campbell RA, Cooper SA, et al. Suppression of postoperative pain by preoperative administration of ibuprofen in comparison to placebo, acetaminophen, and acetaminophen plus codeine. J Clin Pharmacol. 1983;23(1):37-43.
20. Dionne RA. Suppression of dental pain by the preoperative administration of flurbiprofen. Am J Med. 1986;80(3A):41-49.
21. Jackson DL, Moore PA, Hargreaves KM. Preoperative nonsteroidal anti-inflammatory medication for the prevention of postoperative dental pain. J Am Dent Assoc. 1989;119(5):641-647.
22. Gordon SM, Brahim JS, Dubner R, et al. Attenuation of pain in a randomized trial by suppression of peripheral nociceptive activity in the immediate postoperative period. Anesth Analg. 2002;95(5):1351-1357.
23. Moore PA. Long-acting local anesthetics: a review of clinical efficacy in dentistry. Compend Contin Educ Dent. 1990;11(1):22-30.
24. Moore PA, Nahouraii HS, Zovko JG, Wisniewski SR. Dental therapeutic practice patterns in the US. II. Analgesics, corticosteroids, and antibiotics. Gen Dent. 2006;54(3):201-207.
25. Aldous JA, Engar RC. Do dentists prescribe narcotics excessively? Gen Dent. 1996;44(4)332-334.
26. Moore PA, Hersh EV. Combining ibuprofen and acetaminophen for acute pain management after third-molar extractions: translating clinical research to dental practice. J Am Dent Assoc. 2013;144(8):898-908.
27. Cooper SA. Five studies on ibuprofen for postsurgical dental pain. Am J Med. 1984;77(1A):70-77.
28. Hersh EV, Cooper S, Betts N, et al. Single dose and multidose analgesic study of ibuprofen and meclofenamate sodium after third molar surgery. Oral Surg Oral Med Oral Pathol. 1993;76(6):680-687.
29. National Institute on Drug Abuse. DrugFacts: Drug-Related Hospital Emergency Room Visits. http://www.drugabuse.gov/infofacts/HospitalVisits.html. Accessed November 11, 2015.
30. National Institute on Drug Abuse. Media Guide. The Science of Drug Abuse and Addiction: The Basics. http://www.drugabuse.gov/mediaguide/scienceof.html. Accessed November 11, 2015.
31. Golubic S, Moore PA, Katz N, et al. Opioid prescribing in dentistry. Inside Dentistry. 2011;8(6):50-59.
32. McCarthy M. Prescription drug abuse up sharply in the USA. Lancet. 2007;369(9572):1505-1506.
33. Manchikanti L. National drug control policy and prescription drug abuse: facts and fallacies. Pain Physician. 2007;10(3):399-424.
34. Oakley M, O’Donnell J, Moore PA, Martin J. Raising awareness about prescription drug abuse. Inside Dental Assisting. 2012;9(4):18-22.