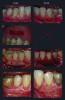
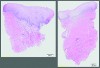
You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
Almost from the first day when dentistry learned to harvest soft tissue grafts from the palate, it began looking for alternative graft sources. This article will review the current understanding about these alternatives, especially as they relate to outcomes, attachment, and stability.
Soft tissue augmentation procedures around teeth generally encompass two goals: keratinized-tissue (KT) width generation and root coverage. Therapies designed to increase KT width are aimed at providing at least the 2 mm of KT (1 mm of which is attached) that is thought to be necessary to maintain periodontal health1 (Figure 1).
When comparing therapies, one should first review the hierarchy of evidence in which findings from systematic reviews and meta-analyses weigh more heavily than those of case reports (Figure 2). When the goal is to increase KT, what evidence is available for the selection of biomaterial alternatives to harvest grafts? Many case reports and case series are available, but to the author’s knowledge, only one systematic review2 has been published. In 2009 Thoma et al2 evaluated studies of allogeneic dermal matrices (ADMs), living cell constructs (LCCs), and other alternative biomaterials compared against autogenous tissue grafts, ie, free gingival grafts (FGGs). The authors found that the variables contributing to success were biomaterial selection, patient characteristics, and surgical techniques. Patient factors were local plaque and tissue biotype, compliance, preference, and expectations. Patient systemic health, including medication use and smoking status, was also an issue. Overall, the “gold standard” therapy for KT generation was autogenous tissue (AT) combined with an apically positioned flap (FGG+APF), which, compared with alternative therapies, significantly improved KT and AT clinical outcomes.
Dentistry in its simplest form focuses on treating teeth, but really it is about treating the patient—and most patients are not as critical as dentists in regards to such matters as “tenths of millimeters.”3 They are more interested in quality-of-life issues such as comfort, cosmetics, and convenience, also known as patient-reported outcomes (PROs). Though the best available evidence indicates that the FGG+APF is most effective clinically, clinicians need to consider their patients’ values and expectations, especially because patients might be willing to live with a little less KT if less morbidity and more comprehensive treatments were involved, ie, a treatment not limited by the amount of harvest graft tissue available (or tolerated by patients), hence the quest for alternatives to harvest grafts.
For the past 20 years the author and his coinvestigator have studied biomaterials that could be considered “nearly as effective” as autogenous graft gold standards but are available in unlimited quantities and meet PRO goals of esthetics and satisfaction. In a recent study,4 the investigators examined an alternative to FGG for generating KT. As with most of their studies, this research was a prospective, randomized, within-subject, controlled (split-mouth), noninferiority comparison trial. They studied 30 patients for 6 months. The primary outcome measure was a change in KT, and the secondary outcomes included traditional clinical measures such as AT and bleeding on probing, but also PRO measures of texture and color match and satisfaction with these therapies.
The investigators studied a xenogeneic collagen matrix (XCM) (Mucograft®, Geistlich Pharma, www.geistlich-na.com) (Figure 3), which is composed of porcine type I and III collagen. The outer layer of XCM contains compact collagen and is designed for tissue adherence and wound protection. The inner matrix layer is a clot-stabilizing macrostructure with a cell-signaling microstructure. Animal studies have shown rapid and organized healing with early vascularization (2 weeks).5,6 Historical collagen research suggests that the combination of intact and fragmented collagen fibrils found in XCM-activated endothelial progenitor cells for angiogenesis and mesenchymal stem cells with anti-inflammatory properties to divert healing away from inflammatory and fibrotic repair toward a more organized, vascularized, and regenerative healing (Figure 4).7-9
As depicted in Figure 5, randomized contralateral teeth with insufficient KT were treated with either control FGG+APF or test XCM+APF therapies. The primary outcome average KT width at 6 months for the two therapies was 2.92 mm ± 0.88 mm for the test XCM+APF and 4.42 mm ± 0.64 mm for the control FGG+APF. Of the 30 test teeth, 29 achieved at least 2 mm of KT. Most importantly, XCM+APF’s color and texture match was superior (Figure 6), and patients preferred the XCM+APF esthetics, while an extensive evaluation of PROs indicated that overall discomfort was reduced by the use of XCM+APF therapy.
The author and his coinvestigator have also examined LCCs (GintuitTM, Organogenesis, Inc., www.organogenesis.com (at the present time this device is no longer available in dentistry) for KT generation.10 In a pivotal multicenter trial, a live sheet of allogeneic fibroblasts and keratinocytes was used to cover the wound beds of apically positioned flaps and acted not as a graft but as a biomodifier “stimulant” for wound healing. A total of 96 patients were examined for 6 months, and again, a suitable, though statistically inferior, amount of KT width was generated (3.21 mm ±1.14 mm for LCC and 4.57 mm ±1.00 mm for FGG). However, color and texture matches were superior with LCC, and, given donor-site morbidity with the control FGG therapy, patients preferred it.
In both the XCM+APF and LCC studies, the harvest graft alternatives generated suitable KT attachment and biopsies showed normal mucoperiosteum with keratinized epithelium (Figure 6).
The remaining question is whether these soft tissue augmentation therapies endure for the long term.11 To answer this, at least 5 years of data is preferable. The good news is that preliminary analysis of LCC and XCM+APF at 3 years have indicated stable results; however, the investigators will follow these patients for 5 years and will publish the results at that time.
To address root coverage therapy, it is necessary to refer to the literature, which has several reviews and meta-analyses. The 1994 European Workshop on Periodontology12 and the 1996 World Workshop in Periodontics Mucogingival Therapy,13 along with systematic reviews from Rocuzzo et al14 and Oates et al15 provide a wide range of results for mean root (43%-96%) and complete root coverage (0%-96%), suggesting once again that not only grafting materials but patient/tooth selection and techniques influence results. Interestingly, the review by Oates et al is the first to note that recession treatment has “mainly been justified by the patient’s wish to improve the esthetic appearance when there is an exposed root.” Surgical technique, especially flap tension (best if 0 g-4 g), flap thickness (best if >0.8 mm), and maintenance of a gingival margin coronal to the cementoenamel junction (CEJ), as well as surgical experience, all contributed to success.
Acellular dermal matrix (ADM) (AlloDerm®, BioHorizons, Inc., www.biohorizons.com) was one of the first harvest graft alternatives tested for root coverage. However, Gapski et al16 in a 2005 meta-analysis found it “difficult to draw anything other than tentative conclusions…primarily because of the weakness in the design and reporting of existing trials.” Harris found that, compared with connective tissue graft (CTG), mean root coverage for ADM did not hold up over time.17 In 2008, when reviewing ADM and guided tissue regeneration with resorbable membranes for root coverage, Chambrone et al18 concluded that, compared with these alternative therapies, CTG+coronally advanced flap (CAF) provided a statistically significant increase in root coverage. Overall comparisons showed CTG+CAF to be the “gold standard” procedure for the treatment of recession defects.
Also in 2008, a root coverage review by Cairo et al19 concluded that only CTG+CAF or enamel matrix derivative (EMD)+CAF could improve outcomes over CAF alone. In each case, CTG and EMD improved KT compared with CAF alone. Most recently, Chambrone et al20 in a 2012 meta-analysis concluded that CTG+CAF, EMD+CAF, and XCM+CAF therapies were superior in achieving complete root coverage compared with CAF alone. A Bayesian network meta-analysis21—in some regard, a meta-analysis of meta-analyses—concluded that CTG+CAF, EMD+CAF, and XCM+CAF therapies could all “be considered very effective combination techniques for treating gingival recessions” (Figure 7).
The author’s own studies22,23 with EMD+CAF and platelet-derived growth factor (PDGF)+CAF have shown good root coverage and patient satisfaction, when compared with CTG+CAF. As with all harvest graft substitutes tested, the learning curve has been less than that required for harvest grafts. With these alternative therapies, the author and his team also have histologic evidence of true regeneration (new cementum and periodontal ligament with inserting collagen fibers and bone)—all without using the palate to harvest grafts.
In the author’s study of XCM+CAF for root coverage,24 the team followed 25 patients for 12 months, again using contralateral, within-subject, matched defects to compare the test therapy against the gold standard CTG+CAF (Figure 8). While recession depth (and hence, root coverage) was the primary efficacy endpoint, the author and his coinvestigator also followed traditional clinical parameters such as KT width, along with PROs such as color/texture esthetics, satisfaction, and pain/discomfort.
At 6 months (and 12 months), results looked esthetically similar to surrounding native tissue, and XCM+CAF yielded an acceptable 88.5% root coverage compared with CTG+CAF (99.3%). Though not equivalent, the XCM+CAF result was more than adequate, particularly when compared with the range of root coverage measures reported in the literature for both the gold standard CTG+CAF and other harvest graft alternatives. Remarkably, no significant difference was observed between KT width generation for the test XCM+CAF or control CTG+CAF (mean change in KT for XCM+CAF was +1.34 mm, and for CTG+CAF +1.26 mm, P = .906). KT width is important not only for long-term health prognosis but also as a predictor for the success of root coverage.25
For comparisons of soft tissue augmentation therapy effectiveness over time, the literature remains the guide. Longer-term data show the success of CAF alone in maintaining complete root coverage is somewhat disappointing at approximately 35%, compared with CTG+CAF at approximately 52%.26 Similarly, ADM+CAF is approximately 35% and apparently no better than CAF alone.19 In the author’s own work (Table 1), complete root coverage for CTG+CAF tends to be 75% to 90% at 10 years. EDM+CAF is maintained at approximately 56% at 10 years, with 83% mean coverage, while PDGF+CAF (data to be published) remains similarly stable at 5 years. XCM+CAF complete root coverage is 71% at 6 months and remains stable at 5 years (data collected and analyzed for journal submission), with no significant change detected over the 5-year interval. Patients were pleased with and preferred this alternative therapy.
In summary, when considering alternative therapies for soft tissue augmentation, clinicians should evaluate outcomes, attachment, and stability and should use the hierarchy of evidence-based studies—always looking for long-term results of at least 5 years. For therapies designed to increase KT around teeth, the clinical outcomes with FGG+APF are the gold standard; however, in the author’s studies, LCC and XCM have provided acceptable clinical results with better PROs for esthetics and patient preference; however, the author must follow these alternative treatments for at least 5 years to confirm their long-term effectiveness. For root coverage therapy, the clinical outcomes with CTG+CAF remain the gold standard; however, EMD+CAF, PDGF+CAF, and XCM+CAF therapies provide more than acceptable results, with equivalent esthetics, and they are patient preferred. EMD+CAF and PDGF+CAF have produced true regeneration, and EMD+CAF, PDGF+CAF, and XCM+CAF have yielded long-term (>5 years) results showing that root coverage can be maintained over time. Notably, in the author’s hands, XCM has proven to be the only harvest graft alternative that can be used successfully either covered (XCM+CAF) or uncovered (XCM+APF). Finally, regardless of the results reviewed and presented here, clinicians should remember that patient, site selection, and surgical technique can influence soft tissue augmentation outcomes, and, as helpful as they might be, literature reviews are no substitute for experience.
About the Author
Michael K. McGuire, DDS
Perio Health Clinical Research Center
Houston, Texas
Queries to the author regarding this course may be submitted to authorqueries@aegiscomm.com.
Acknowledgments
The author wishes to thank his coinvestigator, E. Todd Scheyer, DDS, MS, as well as Rebecca Garcia, RDH, Director of Clinical Research, Perio Health Clinical Research Center (PHCRC), Houston, Texas, for recording outcome measures and coordinating data and Cindy Wainscott, CDA, PHCRC, for study administration.
Conflicts of Interest
Some of the studies reviewed in this article were supported by grants from Geistlich Pharma, Organogenesis, Osteohealth, and Straumann. The author provides lectures sponsored by these companies and has received honoraria from Straumann and Geistlich, but declares no developmental, research, royalty, or other compensatory relationships, and no other conflicts of interest.
References
1. Lang NP, Löe H. The relationship between the width of keratinized gingiva and gingival health. J Periodontol. 1972;43(10):623-627.
2. Thoma DS, Benić GI, Zwahlen M, Hämmerle CH, Jung RE. A systematic review assessing soft tissue augmentation techniques. Clin Oral Implants Res. 2009;20(suppl 4):146-165.
3. Kokich VO Jr, Kiyak HA, Shapiro PA. Comparing the perception of dentists and lay people to altered dental esthetics. J Esthet Dent. 1999;11(6):311-324.
4. McGuire MK, Scheyer ET. A randomized, controlled clinical trial to evaluate a xenogeneic collagen matrix as an alternative to free gingival grafting for oral soft tissue augmentation. J Periodontol. 2014 Mar 5. [Epub ahead of print].
5. Ghanaati S, Schlee M, Webber MJ, et al. Evaluation of the tissue reaction to a new bilayered collagen matrix in vivo and its translation to the clinic. Biomed Mater. 2011;6(1):015010.
6. Rocchietta I, Schupbach P, Ghezzi C, Maschera E, Simion M. Soft tissue integration of a porcine collagen membrane: an experimental study in pigs. Int J Periodontics Restorative Dent. 2012;32(1):e34-e40.
7. Davis GE, Bayless KJ, Davis MJ, Meininger GA. Regulation of tissue injury responses by the exposure of matricryptic sites within extracellular matrix molecules. Am J Pathol. 2000;156(5):1489-1498.
8. Badylak SF, Freytes DO, Gilbert TW. Extracellular matrix as a biological scaffold material: structure and function. Acta Biomater. 2009;5(1):1-13.
9. Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213(2):341-347.
10. McGuire MK, Scheyer ET, Nevins ML, et al. Living cellular construct for increasing the width of keratinized gingiva: results from a randomized, within-patient, controlled trial. J Periodontol. 2011;82(10):1414-1423.
11. Palmer RM, Cortellini P; Group B of European Workshop on Periodontology. Periodontal tissue engineering and regeneration: Consensus Report of the Sixth European Workshop on Periodontology. J Clin Periodontol. 2008;35(8 suppl):83-86.
12. Wennström JL. Mucogingival surgery. In: Lang NP, Karring T, eds. Proceedings of the First European Workshop on Periodontology. Berlin, Germany: Quintessence Publishing Co, Inc.,1994;193-209.
13. Wennström JL. Mucogingival therapy. Ann Periodontol. 1996;1(1):671-701.
14. Roccuzzo M, Bunino M, Needleman I, Sanz M. Periodontal plastic surgery for treatment of localized gingival recessions: a systematic review. J Clin Periodontol. 2002;29(suppl 3):178-194; discussion 195-196.
15. Oates TW, Robinson M, Gunsolley JC. Surgical therapies for the treatment of gingival recession. A systematic review. Ann Periodontol. 2003;8(1):303-320.
16. Gapski R, Parks CA, Wang HL. Acellular dermal matrix for mucogingival surgery: a meta-analysis. J Periodontol. 2005;76(11):1814-1822.
17. Harris RJ. A short-term and long-term comparison of root coverage with an acellular dermal matrix and a subepithelial graft. J Periodontol. 2004;75(5):734-43.
18. Chambrone L, Chambrone D, Pustiglioni FE, et al. Can subepithelial connective tissue grafts be considered the gold standard procedure in the treatment of Miller Class I and II recession-type defects? J Dent. 2008;36(9):659-671.
19. Cairo F, Pagliaro U, Nieri M. Treatment of gingival recession with coronally advanced flap procedures: a systematic review. J Clin Periodontol. 2008;35(8 suppl):83-86.
20. Chambrone L, Pannuti CM, Tu YK, Chambrone LA. Evidence-based periodontal plastic surgery. II. An individual data meta-analysis for evaluating factors in achieving complete root coverage. J Periodontol. 2012;83(4):477-490.
21. Buti J, Baccini M, Nieri M, La Marca M, Pini-Prato GP. Bayesian network meta-analysis of root coverage procedures: ranking efficacy and identification of best treatment. J Clin Periodontol. 2013;40(4):372-386.
22. McGuire MK, Scheyer ET, Schupbach P. Growth factor-mediated treatment of recession defects: a randomized controlled trial and histologic and microcomputed tomography examination. J Periodontol. 2009;80(4):550-564.
23. McGuire MK, Nunn M. Evaluation of human recession defects treated with coronally advanced flaps and either enamel matrix derivative or connective tissue. Part 1: comparison of clinical parameters. J Periodontol. 2003;74(8):1110-1125.
24. McGuire MK, Scheyer ET. Xenogeneic collagen matrix with coronally advanced flap compared to connective tissue with coronally advanced flap for the treatment of dehiscence-type recession defects. J Periodontol. 2010;81(8):1108-1117.
25. Pini Prato G, Rotundo R, Franceschi D, et al. Fourteen-year outcomes of coronally advanced flap for root coverage: follow-up from a randomized trial. J Clin Periodontol. 2011;38(8):715-720.
26. Pini-Prato GP, Cairo F, Nieri M, et al. Coronally advanced flap versus connective tissue graft in the treatment of multiple gingival recessions: a split-mouth study with a 5-year follow-up. J Clin Periodontol. 2010;37(7):644-650.