You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
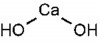
Originally introduced to the field of endodontics by Herman in 1920 as a pulp-capping agent,1 calcium hydroxide [Ca(OH)2] is a white odorless powder with a molecular weight of 74.08 and low solubility in water (approximately 1.2 gL-1 at 25°C), which decreases as the temperature rises (Figure 1).2 The dissociation coefficient of Ca(OH)2 (0.17) permits a slow, controlled release of both calcium and hydroxyl ions. The low solubility is a good clinical characteristic because a long period of time is necessary before it becomes soluble in tissue fluids when in direct contact with vital tissues.3 Ca(OH)2 has a high pH (approximately 12.5 to 12.8), is insoluble in alcohol, and is chemically classified as a strong base. Its main actions come from the ionic dissociation of Ca2+ and OH- ions and their effect on vital tissues, generating the induction of hard tissue deposition and antibacterial activity.2 Ca(OH)2 dissociates into calcium and hydroxyl ions on contact with aqueous fluids.4 In water, Ca(OH)2 has a thixotropic behavior, which means it will be very fluid when agitated.3 When Ca(OH)2 is exposed to carbon dioxide (CO2) or carbonate ions (CO3-) in biologic tissue, the dissociation of the chemical leads to formation of calcium carbonate (CaCO3) and an overall consumption of Ca2+ ions. However, a study showed that after 30 days of exposure to CO2, six preparations of Ca(OH)2 still maintained a purportedly bactericidal pH within the root canal.2
Responsibility of Sealers
It is well understood that when filling root canals with a solid core material, some form of cement is required for a fluid-tight seal that fills the minor gaps between the core material and the dentinal wall of the canal to prevent leakage.5 According to Ørstavik, sealers play an important role in sealing the root canal system by entombing the remaining microorganisms and filling the inaccessible areas of the prepared canals6 (Figure 2). Sealer selection may influence the outcome of endodontic treatment.7
Leakage
A study assessed the apical leakage of four root canal sealers when used with laterally compacted gutta-percha using dye penetration and concluded that the distance dye penetrated the canals was as follows: Apexit® (Ivoclar Vivadent AG, www.ivoclarvivadent.com), 1.67 mm; Sealapex™ (Sybron Dental Specialties Inc., www.sybronendo.com), 2.28 mm; Tubli-Seal™ (Sybron Dental Specialties Inc.), 1.95 mm; AH 26® (Dentsply Limited, www.dentsplymea.com), 0.82 mm; and gutta-percha alone, 8.37 mm.8 It has been revealed that Sealapex had a sealing ability comparable to Tubli-Seal.9 Siqueira et al evaluated the coronal leakage of human saliva into root canals filled using the lateral compaction of gutta-percha and one or another of two Ca(OH)2-based sealers in vitro and found that 35% of the Sealer 26 (Dentsply) samples and 80% of the Sealapex samples were entirely recontaminated at 60 days.10 Using dye penetration methods, Ozata et al compared the apical leakage of Ketac™-Endo (3M ESPE, www.3MESPE.com), Apexit, and Diaket (3M ESPE) and found that there was no significant difference between Apexit and Diaket.11 However, there was significantly more leakage with Ketac-Endo. Timpawat et al concluded that coronal bacterial leakage of canals filled with a Ca(OH)2-based sealer (Apexit) was significantly greater than those filled with a resin-based sealer (AH 26).12 Another study found that the apical sealing ability of Fibrefill™ (Pentron Clinical, www.pentron.com), a resin-based sealer, was significantly better than CRCS® (Coltène/Whaledent Inc, www.coltene.com).13 Cobankara et al showed that the apical sealing ability of Sealapex was significantly better than three other sealers (Rocanal™ 2 [La Maison Dentaire SA, www.rocanol.com], AH Plus® [Dentsply Limited], and RC Sealer [Sun Medical Co, Ltd, www.sunmedical.co.jp/english/index.html]) at 7, 14, and 21 days.14 Pommel et al found that there was no statistically significant difference among AH 26, Pulp Canal Sealer™ (Sybron Dental Specialties Inc.), and Ketac-Endo.15 It was demonstrated that the long-lasting seal of sealers may, among other influencing factors, depend on their thickness and solubility.16
Considering that the main purpose of using sealers is to fill gaps within the irregular root canal system, their solubility and disintegration should be as low as possible. Conversely, to achieve favorable effects, Ca(OH)2 should dissociate into calcium and hydroxyl ions, which is in contrast to the philosophy of using sealers. Therefore, a major dilemma arises regarding both the long-term sealing ability and favorable biological properties of Ca(OH)2-based sealers.
Biocompatibility
There are five approaches to assess the biocompatibility of endodontic materials such as sealers: cytotoxic evaluation, genotoxicity, subcutaneous implants, intraosseous implants, usage tests, and human studies.17 Cytotoxicity is usually assessed on cells such as leukocytes, hela (human cervical carcinoma) cells, and fibroblasts. Cell culture experiments are easier, more rapid, and cheaper than other methods used to test biocompatibility. However, results of these tests cannot be extrapolated to the clinical situation.17 Briseño and Willershausen assessed the cytotoxicity of four different Ca(OH)2-based root canal sealers: Sealapex, Apexit, CRCS, and Endoflas F.S. (Sanlor Laboratories, www.pastafs.com) on human gingival fibroblasts.18 According to their findings, Endoflas F.S. induced a dramatic reduction in the protein synthesis potential of the fibroblasts in the 24-hour group. In the 48-hour group, Endoflas F.S. gave a slightly better response. Endoflas F.S., however, had a significantly higher cytotoxicity with respect to other sealers in both trials. Sealapex demonstrated a relatively low cytotoxicity after 3 days of culturing. Although CRCS had a slightly higher cytotoxicity during the initial phase of the experiments, a declining level of toxicity was measured after 3 days of culturing. Apexit had a relatively high cytotoxicity in the initial phase, but an ascending incorporation rate of L-[14C] leucine in the fibroblasts was found after 3 days of culturing. Leonardo et al evaluated the cytotoxicity of four Ca(OH)2-based sealers and a zinc oxide eugenol (ZOE)-based sealer (Fill Canal® [TechNew, Uredent Dental, www.uredent.com]). They found that the least cytotoxic sealer was Fill Canal, followed in increasing order of cytotoxicity by CRCS, Sealer 26, Apexit, and Sealapex.19 Boiesen and Brodin evaluated the neurotoxic effect of Sealapex and CRCS and found that both materials exhibited reversible and irreversible blocking of nerve conduction after 90-second and 5-minute exposure.20 However, after 30 minutes of contact, the conduction of the compound action potential was irreversibly blocked for both materials. Using hela cells, Miletić et al reported that the toxicity of Apexit was significantly less than AH 26, AH Plus, and Diaket.21 Schwarze et al evaluated the cytotoxicity of several types of root canal sealers in vitro for 1 year using immortalized 3T3 fibroblasts and primary human periodontal ligament fibroblasts.22 The results revealed that pronounced cytotoxic effects were only caused by N2 extracts in both cell cultures. Furthermore, significant cytotoxic alterations were induced by 10-week eluates of Endomethasone (Septodont, www.septodont.com). Other investigated materials did not significantly alter cell metabolism. Eldeniz et al assessed the cytotoxic effects of eight root canal sealers ex vivo: RC Sealer, Epiphany™ (Pentron Clinical), EndoREZ® (Ultradent Products, Inc., www.ultradent.com), GuttaFlow® (Coltène/Whaledent Inc.), Acroseal (Septodont), AH Plus, RoekoSeal (Coltène/Whaledent Inc.), and Apexit, using primary human gingival fibroblasts (HGF) and a mouse fibroblast cell line (L929).23 The results showed that resin-based (Epiphany and EndoREZ) and Ca(OH)2-based (Apexit and Acroseal) sealers were significantly more cytotoxic than other sealers. However, L929 cells were more sensitive to Apexit and EndoREZ than HGF cells. RC Sealer had mild cytotoxicity to HGF cells at both setting times. AH Plus did not exert any cytotoxic effect to HGF cells and aged specimens appeared to induce cellular proliferation. RoekoSeal and GuttaFlow also demonstrated mild cytotoxicity. GuttaFlow was slightly more cytotoxic to both cultures, especially when tested fresh.
In a study to evaluate genotoxicity of Ca(OH)2-based and epoxy-resin–based root canal sealers, Huang et al found that a resin-based sealer produced greater deoxyribonucleic acid damage than a Ca(OH)2-based sealer.24 In a laboratory study to assess the cytotoxicity of Ca(OH)2-based sealers, it was shown that Sealapex was the most cytotoxic sealer followed by CRCS.25 Apexit was the least cytotoxic with the smallest decrease in cell density.25 In a study assessing the tissue toxicity of Grossman’s sealer, eucapercha, Endo-Fill (Dentsply), CRCS, Sealapex, and Hypocal (Ellman, www.ellman.com), the sealers were injected into specific dorsal subdermal tissue sites of 12 guinea pigs.26 Sealapex and Endo-Fill produced less severe inflammatory reactions than any of the other test materials. Grossman’s sealer, CRCS, and Hypocal created principally severe inflammatory responses at both 6 and 15 days, but mild reactions at 80 days. Eucapercha created less severe inflammatory responses than Grossman’s sealer, CRCS, and Hypocal.26
Mittal et al evaluated the tissue toxicity of ZOE, Tubli-Seal, Sealapex, and Endoflas F.S. by injecting them into the subcutaneous connective tissue of the dorsal surface of rats and histologically studying the tissue response.27 According to their findings, Sealapex was associated with the least inflammatory reaction compared to the other sealers used because it caused moderate inflammation at 48 hours that then became mild. Tubli-Seal, Endoflas F.S., and ZOE were highly toxic at 48 hours and at 7 days. This toxicity decreased gradually with time. No inflammatory reaction was seen at 3 months with any of the sealers.
Silva et al evaluated the inflammatory response to Sealapex, CRCS, Apexit, and Sealer 26 in the subcutaneous tissue and in the peritoneal cavity of BALB/c mice.28 The inflammatory response of subcutaneous tissue was analyzed after 2, 4, 8, and 16 days. Intense neutrophilia was seen in response to all of the sealers during the initial periods. Differences among them related to the presence of necrosis and the number of inflammatory cells. In the intermediate phase, a marked differentiation of cells from the mononucleate phagocytic system into macrophages, epithelioid cells, and multinucleate giant cells was observed with Sealapex. This response was less intense with CRCS and Apexit. Tissue necrosis was observed only at the tissue sealer interfaces and only during the initial period with Sealapex but was seen throughout the study with all of the other sealers. The peritoneal cavities of mice were injected with solutions containing the sealers and five mice from each group were killed at 6 hours and 24 hours and at 5 days and at 15 days. During the initial periods (6 hours and 24 hours) there was an intense migration of polymorphonuclear leukocytes to the peritoneal cavity in response to all of the sealers compared to the control. This migration was more intense for Sealer 26 and Apexit. An increase in mononucleate cell number was observed after 6 hours and 24 hours and 5 days for all of the sealers, and no differences were observed in relation to the control after 15 days.
Kolokouris et al evaluated the in-vivo biocompatibility of Apexit and Pulp Canal Sealer after implantation in rat connective tissue.29 The findings revealed that severe inflammatory reactions with differing extensions of necrosis were observed with Apexit on days 5 and 15. The intensity of the reaction had diminished by day 60, and this reduction continued progressively through day 120. The reaction was characterized by the presence of connective tissue with a few macrophages. Moderate to severe inflammation with confined areas of necrosis was observed in the Pulp Canal Sealer specimens on day 5. The intensity of the reaction diminished by days 15, 60, and 120, but remained greater than Apexit through long-term observation. Figueiredo et al evaluated tissue response to four endodontic sealers (N-Rickert [Biodinâmica, www.biodinamica.com.br], AH 26, Fill Canal, and Sealer 26) placed in the oral mucosa of rabbits by either submucous injection or implant in polyethylene tubes.30 The findings demonstrated that there was no difference between the two methods of implantation. In addition, all of the sealers elicited some kind of inflammatory response. Fill Canal was the most irritating, followed by N-Rickert and AH 26. Sealer 26 elicited only a mild reaction. Bernáth and Szabó evaluated the type and degree of inflammatory reaction initiated by four sealers (AH 26, Apexit, Endomethasone, and Grossman’s sealers) by overfilling the root canals in the teeth of monkeys.31 The result of the treatment was evaluated after 6 months by histological assessment of the periapical tissues. In the group of root canals filled within the root, no inflammatory reaction was detected in specimens with Apexit and Grossman’s sealers, but the other two sealers initiated different degrees of lymphocytic/plasmocytic tissue reactions. Endomethasone produced a mild lymphocytic/plasmocytic reaction in three of the nine cases, and AH 26 caused mild lymphocytic/plasmocytic infiltration in two of the seven cases. In the group with overfilled root canals, all four sealers produced inflammatory reactions. The periapical tissue reactions of overfilled root canals were similar to the reactions found in the cases filled within the canal. However, additional histological features developed in the specimens of Endomethasone and AH 26. Endomethasone initiated a foreign body-type granulomatous reaction around the sealer particles and AH 26 particles were engulfed by macrophages. The overfilled root canals with Apexit and Grossman’s sealers caused only lymphocytic/plasmocytic reactions.
Antibacterial Activity
Microorganisms infecting the root canal dentin might adhere superficially to the dentinal wall or penetrate deeper into the dentinal tubules.32,33 It might be expected that superficially adhering bacteria would be killed more readily than those shielded in the depths of dentinal tubules, but microorganisms inside the dentinal tubules can also be challenged by antimicrobial components leaching from sealers. Therefore, antimicrobial testing of sealers should take into consideration these two effects of sealer contact with microorganisms.34 Two main methods have been used to study the antimicrobial effects of Ca(OH)2-based sealers: agar diffusion tests (ADT) and direct contact tests (DCT).
Studies Using ADT
An ADT study showed that Roth sealer (a ZOE-based sealer) (Roth International Ltd., www.rothendo.com) inhibited the growth of Streptococcus anginosus (milleri) more effectively than several Ca(OH)2-based sealers (Sealapex, Apexit, and CRCS).35 In another ADT study, Mickel et al evaluated the antimicrobial activity of four root canal sealers on Enterococcus faecalis.36 A statistically significant difference was observed between all four groups of sealers. Roth 811 had the largest zone of inhibition (1.1 mm), followed by Sealapex (0.8 mm), and Kerr Pulp Canal EWT (EndoTech Inc., www.endo-tech.com) (0.5 mm). AH Plus exhibited no antimicrobial activity. Abdulkader et al evaluated the effect of several sealers against Capnocytophaga ochracea, Porphyromonas gingivalis, and Peptostreptococcus micros using ADT.37 They found that the products that inhibited bacterial growth (in descending order) were: Roth sealer, Ketac-Endo, Tubli-Seal, Apexit, and Sealapex. A study by al-Khatib et al assessed the antibacterial effect of Grossman’s sealer, Tubli-Seal, Calciobiotic (Coltène/Whaledent Inc), Sealapex, Hypocal, eucapercha, Nogenol™ (GC America Inc., www.gcamerica.com), and AH 26 against Streptococcus mutans, Staphylococcus aureus, and Bacteroides endodontalis using ADT.38 The results demonstrated that Grossman’s sealer had the greatest overall antibacterial activity. However, AH 26 exhibited the greatest antibacterial activity against B. endodontalis. The ZOE-based sealers exhibited more antimicrobial activity than either the Ca(OH)2-based sealers or eucapercha. Using ADT, Lai et al found that the antibacterial activity of zinc oxide-based and resin-based sealers were more than Sealapex (a Ca(OH)2-based sealer).39
Studies Using DCT
There are fewer studies using DCT. Heling and Chandler as well as Saleh et al demonstrated that Sealapex as well as Apexit had less antibacterial efficacy than resin-based and zinc oxide-based sealers.40,41 Furthermore, these two studies demonstrated that the antimicrobial effect of Ca(OH)2-based sealers increased with time, most likely because of the disintegration of the sealer and an increase in the amount of hydroxyl ions over time. Kayaoglu et al showed that Ca(OH)2-based sealers (Sealapex and Apexit) were ineffective in killing bacteria in the short term (24 hours).34 According to Cobankara et al, Ketac-Endo and AH Plus were more potent bacterial growth inhibitors than Sealapex.42
Duarte et al evaluated the pH and calcium ion release of three root canal sealers (Sealapex, Sealer 26, and Apexit) at 24 hours and 48 hours, and at 7 days and 30 days after spatulation.43 Sealapex produced an alkaline pH and released significantly greater amounts of calcium, with even more pronounced results after 30 days. Furthermore, Sealapex had the highest calcium and hydroxyl release, especially after longer time intervals, whereas Sealer 26 had the highest release during the initial periods (ie, during its setting period). Apexit showed the least satisfactory results.
Solubility
When considering the solubility of endodontic sealers, it should be noted that their solubility in a specific solvent (eg, chloroform) is a positive characteristic, whereas their solubility in tissue fluids is a negative characteristic.
Solubility in Tissue Fluids
Ca(OH)2-based sealers were introduced in an attempt to stimulate periapical healing with bone repair through the release of Ca(OH)2.44 According to Esberard et al, Ca(OH)2-based sealers release OH- and Ca2+ ions.45 These sealers evoke an increase of pH when placed in distilled water (48 hours after setting) of 9.14 and 8.6. Under the same conditions, pure Ca(OH)2 paste increased the pH to 12.5. Sleder et al demonstrated that Sealapex had no greater dissolution (based upon linear penetration) than Tubli-Seal at both 2 weeks and 32 weeks.46 They concluded that Sealapex could withstand long-term exposure to tissue fluids without significant leakage. Tronstad et al assessed the solubility of CRCS and Sealapex in dog teeth and found that CRCS was more stable than Sealapex.47 McMichen et al reported that the solubility of Apexit in water was significantly more than AH Plus, Tubli-Seal EWT, and Endion (VOCO GmbH, www.voco.com).48
Solubility in Solvents
A requirement of root canal re-treatment is the removal of endodontic filling materials from the root canal.49 Various solvents for dissolving root canal filling material have been studied.50,51 Chloroform is the most common solvent used to remove root filling materials (ie, gutta-perch and sealers).52 Benzene and xylene, which are effective solvents, may be potential carcinogens.53 Halothane, another solvent, is highly volatile.54 The high cost and volatility of halothane and its potential for inducing idiosyncratic hepatic necrosis makes it a less desirable solvent.54 Whitworth and Boursin evaluated the effect of two volatile solvents (chloroform and halothane) on the solubility of root canal sealers (Ketac-Endo, Tubli-Seal EWT, Apexit, and AH Plus).55 Their findings showed that Ketac-Endo was the least soluble in chloroform and halothane, with < 1% weight loss after 10 minutes of exposure to either solvent. Apexit had low solubility with 11.6% and 14.19% weight loss after 10 minutes of exposure to chloroform and halothane, respectively. The difference between solvents was not significant. Tubli-Seal EWT was significantly less soluble in halothane than chloroform (5.19% and 62.5% weight loss after 10 minutes of exposure, respectively). Its solubility in halothane was not significantly different from that of Apexit. AH Plus was significantly more soluble than all of the other materials in both chloroform and halothane (96% and 68% weight loss after 10 minutes of exposure, respectively). Schäfer and Zandbiglari showed that Ca(OH)2-based sealers demonstrated higher solubility in chloroform than in eucalyptus oil.56 Keleş and Köseoğlu found that the solubility of a Ca(OH)2-based sealer in sodium hypochlorite and ethylenediaminetetraacetic acid was significantly greater than ZOE-based, silicone-based, and resin-based sealers.54 However, its solubility was similar to polyketone. Martos et al evaluated the solubility of a Ca(OH)2-based (Sealer 26), a silicon polydimethylsiloxane-based sealer (RoekoSeal), and ZOE-based sealers (Endo-Fill, and Intrafill [SS White, www.sswhite.com]) in eucalyptol, xylol, orange oil, and distilled water.57 Their findings revealed that xylol and orange oil had similar effects, with significant solubilization of the cements tested. Endo-Fill and Sealer 26 did not have any significant difference in solubilization at the two immersion times, whereas RoekoSeal and Intrafill had more pronounced solubility at 10 minutes. The lowest levels of solubilization occurred in RoekoSeal, Sealer 26, Endo-Fill, and Intrafill.
Toxicity
It has been reported that Sealapex caused a moderate-to-severe inflammatory reaction, whereas CRCS caused a mild to moderate reaction in rat connective tissue.58 Kolokouris et al evaluated the in-vivo biocompatibility of Apexit and Pulp Canal Sealer after implantation in rat connective tissue at 5-, 15-, 60-, and 120-day intervals.29 Severe inflammatory reactions occurred with differing levels of necrosis with Apexit on the 5th and 15th days. The intensity of the reaction had diminished by the 60th day, and this reduction continued progressively to the 120th day. It was characterized by the presence of connective tissue with a few macrophages. Moderate to severe inflammation with confined areas of necrosis was observed in the Pulp Canal Sealer specimens on the 5th day. The intensity of the reaction diminished by the 15th, 60th, and 120th days, but remained marginally greater than Apexit through long-term observation periods.
Osorio et al reported that CRCS was well tolerated by human gingival fibroblasts and L-929 cells.59 Leonardo et al found that the cytotoxicity of four Ca(OH)2-based sealers (Sealapex, CRCS, Apexit, and Sealer 26) was more pronounced than a ZOE-based sealer (Fill Canal, Dermo Laboratório Ltda).60 Camps and About concluded that the high cytotoxicity of Sealapex did not decrease over time.61 Soares et al found that overfilled canals containing Ca(OH)2-based sealers caused chronic inflammatory reactions in the periapical tissues of dog teeth.62
Conclusion
The sealing ability of Ca(OH)2-based sealers compared to other sealers is ambiguous. This may be a result of factors such as the method used to evaluate leakage and the often limited sample sizes included in such studies. However, it is clear that there is no superiority for Ca(OH)2-based sealers over other groups of sealers. Some controversies regarding the biocompatibility of Ca(OH)2-based sealers could be attributed to the evaluation method. However, most studies concluded that the biocompatibility of Ca(OH)2-based sealers were within an acceptable range compared to other root canal sealers. The antibacterial activity of Ca(OH)2-based sealers is lower than other similar materials, especially ZOE-based and resin-based sealers. Because of the low number of studies, the solubility of Ca(OH)2-based sealers compared to other sealers in tissue fluids is not known. The solubility rate of Ca(OH)2-based sealers compared to other sealers in solvents is still controversial. Although Ca(OH)2 paste is well tolerated by periapical tissues, it has a detrimental effect on periodontal tissues when used as an intracanal medicament. The biocompatibility of Ca(OH)2-based sealers is controversial.
About the Authors
Zahed Mohammadi, DMD, MSD
Associate Professor, Iranian Center for Endodontic Research,
Shaheed Beheshti University of Medical Sciences, Tehran, Iran;
Iranian National Elite Foundation, Tehran, Iran
Mohammad Karim Soltani, DMD, MSD
Assistant Professor, Department of Orthodontics,
Hamedan University of Medical Sciences, Hamedan, Iran
Sousan Shalavi, DMD
Private Practice, Hamedan, Iran
Mohammad Yazdizadeh, DMD, MSD
Assistant Professor, Department of Endodontics,
Ahvaz University of Medical Sciences, Ahvaz, Iran
Mansour Jafarzadeh, DMD
Assistant Professor, Department of Endodontics,
Ahvaz University of Medical Sciences, Ahvaz, Iran
Queries to the author regarding this course may be submitted to authorqueries@aegiscomm.com.
References
1. Hermann BW. Calcium hydroxid als Mittelzurn, Behandeln und Fullen von Wurzelkanalen [thesis]. Germany: University of Würzburg; 1920.
2. Farhad A, Mohammadi Z. Calcium hydroxide: a review. Int Dent J. 2005;55(5):293-301.
3. Spångberg L, Haapasalo M. Rationale and efficacy of root canal medicaments and root filling materials with emphasis on treatment outcome. Endod Topics. 2002;2(1):35-58.
4. Rehman K, Saunders WP, Foye RH, Sharkey SW. Calcium ion diffusion from calcium hydroxide-containing materials in endodontically-treated teeth: an in vitro study. Int Endod J. 1996;29(4):271-279.
5. Grossman LI. An improved root canal cement. J Am Dent Assoc. 1958;56(3):381-385.
6. Ørstavik D. Materials used for root canal obturation: technical, biological and clinical testing. Endod Topics. 2005;12(1):25-38.
7. Ørstavik D, Kerekes K, Erkisen HM. Clinical performance of three endodontic sealers. Endod Dent Traumatol. 1987;3(4):178-186.
8. Limkangwalmongkol S, Abbott PV, Sandler AB. Apical dye penetration with four root canal sealers and gutta-percha using longitudinal sectioning. J Endod. 1992;18(11):535-539.
9. Sleder FS, Ludlow MO, Bohacek JR. Long-term sealing ability of a calcium hydroxide sealer. J Endod. 1991;17(11):541-543.
10. Siqueira JF Jr, Rôças IN, Lopes HP, de Uzeda M. Coronal leakage of two root canal sealers containing calcium hydroxide after exposure to human saliva. J Endod. 1999;25(1):14-16.
11. Ozata F, Onal B, Erdilek N, Türkün SL. A comparative study of apical leakage of Apexit, Ketac-Endo, and Diaket root canal sealers. J Endod. 1999;25(9):603-604.
12. Timpawat S, Amornchat C, Trisuwan WR. Bacterial coronal leakage after obturation with three root canal sealers. J Endod. 2001;27(1):36-39.
13. Economides N, Kokorikos I, Kolokouris I, et al. Comparative study of apical sealing ability of a new resin-based root canal sealer. J Endod. 2004;30(6):403-405.
14. Cobankara FK, Orucoglu H, Sengun A, Belli S. The quantitative evaluation of apical sealing of four endodontic sealers. J Endod. 2006;32(1):66-68.
15. Pommel L, About I, Pashley D, Camps J. Apical leakage of four endodontic sealers. J Endod. 2003;29(3):208-210.
16. Georgopoulou MK, Wu MK, Nikolaou A, Wesselink PR. Effect of thickness on the sealing ability of some root canal sealers. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;80(3):338-344.
17. Hauman CH, Love RM. Biocompatibility of dental materials used in contemporary endodontic therapy: a review. Part 1. Intracanal drugs and substances. Int Endod J. 2003;36(2):75-85.
18. Briseño BM, Willershausen B. Root canal sealer cytotoxicity with human gingival Fibroblasts. III. Calcium hydroxide-based sealers. J Endod. 1992;18(3):110-113.
19. Leonardo MR, Silva LAB, Leonardo RT. Tratamento de canal radicular em sessão única: crença vs. ciência. In: Feller C, Gorab R, eds. Atualização na Clínica Odontológica. São Paulo: Artes Médicas; 2000:29-57.
20. Boiesen J, Brodin P. Neurotoxic effect of two root canal sealers with calcium hydroxide on rat phrenic nerve in vitro. Endod Dent Traumatol. 1991;7(6):242-245.
21. Miletić I, Anić I, Karlović Z, et al. Cytotoxic effect of four root filling materials. Endod Dent Traumatol. 2000;16(6):287-290.
22. Schwarze T, Leyhausen G, Geurtsen W. Long-term cytocompatibility of various endodontic sealers using a new root canal model. J Endod. 2002;28(11):749-753.
23. Eldeniz AU, Mustafa K, Ørstavik D, Dahl JE. Cytotoxicity of new resin-, calcium hydroxide- and silicone-based root canal sealers on fibroblasts derived from human gingiva and L929 cell lines. Int Endod J. 2007;40(5):329-337.
24. Huang FM, Tai KW, Chou MY, Chang YC. Cytotoxicity of resin-, zinc oxide-eugenol-, and calcium hydroxide-based root canal sealers on human periodontal ligament cells and permanent V79 cells. Int Endod J. 2002;35(2):153-158.
25. Beltes P, Koulaouzidou E, Kotoula V, Kortsaris AH. In vitro evaluation of the cytotoxicity of calcium hydroxide-based root canal sealers. Endod Dent Traumatol. 1995;11(5):245-249.
26. Yesilsoy C, Koren LZ, Morse DR, Kobayashi C. A comparative tissue toxicity evaluation of established and newer root canal sealers. Oral Surg Oral Med Oral Pathol. 1988;65(4):459-467.
27. Mittal M, Chandra S, Chandra S. Comparative tissue toxicity evaluation of four endodontic sealers. J Endod. 1995;21(12):622-624.
28. Silva LA, Leonardo MR, Faccioli LH, Figueiredo F. Inflammatory response to calcium hydroxide based root canal sealers. J Endod. 1997;23(2):86-90.
29. Kolokouris I, Economides N, Beltes P, Vlemmas I. In vivo comparison of the biocompatibility of two root canal sealers implanted into the subcutaneous connective tissue of rats. J Endod. 1998;24:82-85.
30. Figueiredo JA, Pesce HF, Gioso MA, Figueiredo MA. The histological effects of four endodontic sealers implanted in the oral mucosa: submucous injection versus implant in polyethylene tubes. Int Endod J. 2001;34(5):377-385.
31. Bernáth M, Szabó J. Tissue reaction initiated by different sealers. Int Endod J. 2003;36(4):256-261.
32. Ando N, Hoshino E. Predominant obligate anaerobes invading the deep layers of root canal dentin. Int Endod J. 1990;23(1):20-27.
33. Peters OA, Laib A, Göhring TN, Barbakow F. Changes in root canal geometry after preparation assessed by high resolution computed tomography. J Endod. 2001;27(1):1-6.
34. Kayaoglu G, Erten H, Alaçam T, Ørstavik D. Short-term antibacterial activity of root canal sealers towards Enterococcus faecalis. Int Endod J. 2005;38(7):483-488.
35. Mickel AK, Wright ER. Growth inhibition of Streptococcus anginosus (milleri) by three calcium hydroxide sealers and one zinc oxide-eugenol sealer. J Endod. 1999;25(1):34-37.
36. Mickel AK, Nguyen TH, Chogle S. Antimicrobial activity of endodontic sealers on Enterococcus faecalis. J Endod. 2003;29(4):257-258.
37. Abdulkader A, Duguid R, Saunders EM. The antimicrobial activity of endodontic sealers to anaerobic bacteria. Int Endod J. 1996;29(4):280-283.
38. al-Khatib ZZ, Baum RH, Morse DR, et al. The antimicrobial effect of various endodontic sealers. Oral Surg Oral Med Oral Pathol. 1990;70(6):784-790.
39. Lai CC, Huang FM, Yang HW, et al. Antimicrobial activity of four root canal sealers against endodontic pathogens. Clin Oral Investig. 2001;5(4):236-239.
40. Heling I, Chandler NP. The antimicrobial effect within dentinal tubules of four root canal sealers. J Endod. 1996;22(5):257-259.
41. Saleh IM, Ruyter IE, Haapasalo M, Ørstavik D. Survival of Enterococcus faecalis in infected dentinal tubules after root canal filling with different root canal sealers in vitro. Int Endod J. 2004;37(3):193-198.
42. Cobankara FK, Altinöz HC, Ergani O, et al. In vitro antibacterial activities of root-canal sealers by using two different methods. J Endod. 2004;30(1):57-60.
43. Duarte MA, Demarchi AC, Giaxa MH, et al. Evaluation of pH and calcium ion release of three root canal sealers. J Endod. 2000;26(7):389-390.
44. Bergenholtz G, Hørsted-Bindslev P, Reit C. Textbook of Endodontology. 1st ed. Hoboken, NJ: Wiley-Blackwell; 2003:261-285.
45. Esberard RM, Carnes DL Jr, Del Rio CE. pH changes at the surface of root dentin when using root canal sealers containing calcium hydroxide. J Endod. 1996;22(8):399-401.
46. Sleder FS, Ludlow MO, Bohacek JR. Long-term sealing ability of a calcium hydroxide sealer. J Endod. 1991;17(11):541-543.
47. Tronstad L, Barnett F, Flax M. Solubility and biocompatibility of calcium hydroxide-containing root canal sealers. Endod Dent Traumatol. 1988;4(4):152-159.
48. McMichen FR, Pearson G, Rahbaran S, Gulabivala K. A comparative study of selected physical properties of five root-canal sealers. Int Endod J. 2003;36(9):629-635.
49. Mandel E, Friedman S. Endodontic retreatment: a rational approach to root canal reinstrumentation. J Endod. 1992;18(11):565-569.
50. Olsson B, Sliwkowski A, Langeland K. Intraosseous implantation for biological evaluation of endodontic materials. J Endod. 1981;7(6):253-265.
51. Barbosa SV, Burkard DH, Spångberg LS. Cytotoxic effects of gutta-percha solvents. J Endod. 1994;20(1):6-8.
52. Wilcox LR. Endodontic retreatment with halothane versus chloroform solvent. J Endod. 1995;21(6):305-307.
53. Lynge E, Anttila A, Hemminki K. Organic solvents and cancer. Cancer Causes Control. 1997;8(3):406-419.
54. Keleş A, Köseoğlu M. Dissolution of root canal sealers in EDTA and NaOCl solutions. J Am Dent Assoc. 2009;140(1):74-79.
55. Whitworth JM, Boursin EM. Dissolution of root canal sealer cements in volatile solvents. Int Endod J. 2000;33(1):19-24.
56. Schäfer E, Zandbiglari T. A comparison of the effectiveness of chloroform and eucalyptus oil in dissolving root canal sealers. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;93(5):611-616.
57. Martos J, Gastal MT, Sommer L, et al. Dissolving efficacy of organic solvents on root canal sealers. Clin Oral Investig. 2006;10(1):
50-54.
58. Economides N, Kotsaki-Kovatsi VP, Poulopoulos A, et al. Experimental study of the biocompatibility of four root canal sealers and their influence on the zinc and calcium content of several tissues. J Endod. 1995;21(3):122-127.
59. Osorio RM, Hefti A, Vertucci FJ, Shawley AL. Cytotoxicity of endodontic materials. J Endod. 1998;24(2):91-96.
60. Leonardo RT, Consolaro A, Carlos IZ, Leonardo MR. Evaluation of cell culture cytotoxicity of five root canal sealers. J Endod. 2000;26(6):328-330.
61. Camps J, About I. Cytotoxicity testing of endodontic sealers: a new method. J Endod. 2003;29(9):583-586.
62. Soares I, Goldberg F, Massone EJ, Soares IM. Periapical tissue response to two calcium hydroxide-containing endodontic sealers. J Endod. 1990;16(4):166-169.