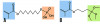You must be signed in to read the rest of this article.
Registration on CDEWorld is free. Sign up today!
Forgot your password? Click Here!
Metals, polymers, ceramics, and composites are used in dentistry to restore teeth. Ceramics are used for many dental applications ranging from the glass in clinicians’ loupes to the filler in restorative resins. Ceramics are characterized by their hardness, brittleness, thermal and electrical insulation, and biocompatibility. The ceramics most commonly used in dentistry are oxides, particularly silicon dioxide (SiO2), aluminum oxide (Al2O3), and zirconium dioxide (ZrO2). The nomenclature for naming oxide ceramics is achieved by removing the suffix of the metallic atom and replacing it with –a; for example, silicon dioxide becomes silica. This article reviews the microstructure of current ceramic materials and how it relates to their mechanical properties, clinical techniques, and optical properties. Typical ceramics currently in use are described and their clinically relevant properties compared.
Overview
Dental ceramics can be divided into primarily glass-containing (ie, feldspathic porcelain), reinforced glass (ie, leucite and lithium disilicate), and crystalline (ie, zirconia and alumina). The most frequent clinical failure of bilayered all-ceramic restorations is chipping of veneering porcelain caused by core–veneer coefficient of thermal expansion (CTE) mismatch, surface grinding, inadequate core design, or overloading. Monolithic crystalline ceramic crowns have a smaller incidence of fracture, because these ceramic materials are stronger than reinforced glass ceramics. Crystalline (zirconia and alumina) and reinforced glass ceramics (lithium disilicate) produce less opposing enamel wear than veneered porcelain. Polishing ceramic restorations after occlusal adjustments typically produces less opposing wear than staining or glazing the restoration.
Ceramic crowns can either be traditionally cemented or adhesively bonded depending upon several factors, including: the strength of the ceramic used; the retentiveness of the preparation; whether the preparation is in dentin or enamel; and the ability to isolate. Porcelain and reinforced glass ceramics should be etched with hydrofluoric acid (HF) and silanated prior to bonding. Zirconia and alumina crowns should be tribochemically coated, 10-methacryloyloxydecyl dihydrogen phosphate (MDP)-primed, or both prior to adhesive bonding. Bonding with resin cements produces a higher bond of dentin to porcelain and glass ceramics than traditional cementing with resin-modified glass ionomer. Cementing untreated zirconia to dentin with resin-modified glass ionomer or many resin cements produces a similar bond strength. Adhesive resin bonding strengthens feldspathic porcelain but not necessarily glass ceramics or polycrystalline ceramics.
Ceramic Microstructure
Ceramics are composed of a metal and non-metal element. In a liquid state, these elements are freely moving. Upon solidification, these elements can either arrange themselves in an ordered structured crystal or into an amorphous unstructured glass. Generally, cooling a ceramic material slowly will allow it time to solidify into a crystal, whereas cooling it rapidly forces the atoms into random orientations as seen in a glass (Figure 1, elements A and B). The process of heating a crystalline or partially crystalline ceramic and then rapidly cooling it, thereby creating a glassy coating, is termed vitrification. A crown can be intentionally vitrified in order to self-glaze its surface or unintentionally de-vitrified during the fabrication process, increasing its opacity.
The microstructure of the ceramic determines its mechanical and optical properties. Crystalline ceramics have atoms arranged into closely packed crystals with a high atomic density; glasses have a lower atomic density. Therefore, a crack propagating through a crystalline ceramic will have to break more atomic bonds per unit area than a crack travelling through the same unit area of a glass ceramic. Thus, crystalline ceramics are generally stronger than glass ceramics. The lower atomic density of glass also allows light to pass through it, which causes it to be translucent. Crystal ceramics, conversely, are typically opaque. There are some exceptions (such as cubic zirconia or quartz), where the crystalline microstructure of the ceramic corresponds to the wavelength of light and the crystal is translucent. In summary, assuming proper processing techniques are used, higher crystal content of a ceramic generally contributes to higher strength and decreased translucency.
The crystal content of a ceramic also affects its CTE, and, resultantly, crystal content is used to match the CTE of a veneering ceramic to its supporting core material. Crystals have a lower CTE than glasses. Additionally, ceramics are stronger in compression than in tension or shear. Therefore, veneering ceramics need to have a lower CTE than the core material they are covering in order to place the veneering ceramic in compression. When fabricating a bilayered crown, the core and veneer material fuse at the melting temperature of the veneer. As the two materials cool, the veneering ceramic (with a lower CTE) will shrink less than the core material, causing the core material to squeeze the veneer with compressive force. The greater shrinkage of the core material places the veneer under compression, thus strengthening it.
Although it is helpful to generalize ceramics as either crystals or glasses to explain their physical properties, in reality most ceramics have both crystal and glass phases (Figure 1, element C). Various types of ceramics are used in current dental prostheses.
Types of Ceramics
Current dental ceramics can be classified in three categories: porcelain, which contains mostly glass phase; glass ceramics, which have a high concentration of reinforcing crystal content; and polycrystalline ceramics, which are composed of mostly crystals. Strength and toughness values of each category are presented in Table 1.
Porcelain—Dental porcelain is the most translucent type of ceramic and is typically used for esthetic applications such as veneers or veneering core materials. Dental porcelain, also called feldspathic porcelain, is a specific type of ceramic composed of feldspar, kaolinite, and quartz. The feldspar contributes the glassy matrix of the porcelain, and the kaolinite and quartz contribute reinforcing crystals of alumina and silica. Since porcelain is also the weakest ceramic, it is either used as a veneer of a stronger core material or chemically bonded to the underlying tooth to increase its strength. When used to veneer a core material, the crystal content and CTE of the porcelain is adjusted to match the CTE of the material it is covering (ie, higher crystal content and lower CTE when veneering zirconia than metal).
Glass ceramics—Ceramics in this category contain a high concentration of reinforcing crystals. Crystals embedded within a glass matrix help to deflect cracks and therefore strengthen the ceramic.1 Leucite (KAlSi2O6) crystals are used to strengthen feldspathic porcelain while maintaining its translucency and decreasing its CTE. Lithium disilicate (LiS2O5) is a needle-shaped crystal composed of 30% amorphous silica and 70% crystalline lithium-disilicate crystals with increased flexural strength but decreased translucency compared to feldspathic porcelain. These materials provide a balance between strength and translucency that allows them to be used as monolithic (single-layered) prostheses for restoration of anterior teeth.
Polycrystalline ceramics—The last category of dental ceramics is polycrystals. The two common polycrystals (also called metal ceramics) are alumina and zirconia. In these materials, zirconium or aluminum and oxygen atoms arrange in specific crystal patterns. A uniform arrangement of atoms in a given crystal pattern forms a crystalline grain, and a bulk piece of zirconia or alumina contains many crystalline grains. A unique property of zirconia is that it undergoes transformation toughening. In this process, grains of the more compact tetragonal crystal structure of zirconia expand into the monoclinic phase, induced by a propagating crack. The expansion causes compression and halting of the crack.2 Polycrystals are the most opaque and strongest class of ceramics and have been typically used as core materials veneered with porcelain or for monolithic posterior crowns. Current research to produce more translucent forms of these materials by manipulating grain size and varying doping agents to produce more esthetic monolithic restorations is underway.3
Resin ceramics represent a new category of ceramics. These materials contain a high content of ceramic particles (more than 80% wt) in a resin matrix and are essentially very highly filled resin composites that offer a high surface polish, high elasticity, and shorter milling times.
Clinically Relevant Properties of Dental Ceramics
In this section, clinically relevant properties of dental ceramics are addressed, and a comparison of these properties between classes of materials is provided.
Strength and Fracture Properties
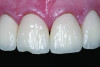
Ceramics are brittle, meaning that they tend to fracture without significant deformation (ie, high strength but relatively low toughness). Failures are more frequent in the veneering material (Figure 2), as reported in a recent systematic review of 3-year clinical trials of zirconia fixed partial dentures (FPDs), which found a 27% chipping occurrence in the veneering porcelain and only a 1% incidence of fracture of the framework.4 The severity of the veneer fracture will determine whether the defect can be polished, repaired with composite, or if the restoration requires replacement. Reasons for replacing the restoration include fractures that extend to a functional area (ie, occlusal contact or supporting a connector) or produce unacceptable anatomic contours or esthetics.5
Veneer fracture typically originates either in the veneering ceramic or at the core–veneer interface. An in-vitro study of lithium-disilicate crowns loaded to fracture discovered that the failure mode was 75% core–veneer interfacial, 20% within the veneering material, and 5% core fractures.6 Fractographic analysis of 19 failed veneered zirconia crowns found 10 fractures originating from the surface of the veneer and six originating from the core–veneer interface.7 Interfacial fractures of bilayered all-ceramic restorations have been attributed to tensile preloads in the veneer created by mismatched CTEs of the core and veneer ceramics. Adjusting the CTE of the veneering ceramic can help improve the bond between the veneer and the core.8,9 Another source of interfacial failure is the bond between the veneer and core material. Unfortunately, this bond is poorly understood and may develop from mechanical or chemical bonding.10 Surface treatments (particle-abrasion and liners) have been developed to improve the bond, however studies11-13 have reported that these techniques provide limited improvement. Cohesive failure within the veneering material results from inadequate core support of the veneer, overloading of the crown, and surface defects from adjustments.7 Cores that are designed to support an even layer of veneering ceramic (particularly at cusps and marginal ridges) perform better than cores of uniform thickness.14,15 Anatomic cores increase the fracture strength of the restoration by 30%.16 Based on this information, clinical recommendations to prevent fracture include proper occlusal adjustment and polishing with a heatless stone and rubber polisher, as well as ensuring that the laboratory has adequately designed an anatomical core framework and chosen a veneering porcelain that matches the core CTE.
Recently, monolithic or full-contour restorations have become popular because they avoid porcelain veneer chipping. The choice of material for a monolithic restoration is partially based on the strength of the ceramic and the amount of tooth reduction that is possible. Lin reported that the biaxial flexural strength of ceramics increases with increased crystal content: 163.95 MPa for leucite, 365.06 MPa for lithium disilicate, and 1,039.71 MPa for zirconia.17 Based on these differences, manufacturers have recommended axial tooth reduction for posterior monolithic crowns of 1.5 mm for lithium disilicate and 0.6 mm for zirconia. An in-vitro test reported an ultimate crown fracture strength of 1,668 MPa for 0.6 mm uniform monolithic zirconia, 2,026 MPa for 1.5 mm uniform monolithic lithium disilicate, and 1,465 MPa for 1.2 mm uniform monolithic lithium disilicate after thermocycling and load cycling.18 When preparing full-contour crowns, it is therefore important to ensure proper tooth reduction for the selected restorative material.
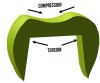
Fractures that initiate in the core material arise from radial cracks on the internal surface of the crown.19 Ceramics are subjected to tensile forces at the internal surfaces of crowns, making them more susceptible to fracture in this area (Figure 3). Grinding the ceramic core with a rough diamond has shown to decrease the flexural strength of the material,20 so clinicians should adjust the tooth preparation instead of the intaglio surface of the ceramic crown when fitting an all-ceramic crown. Another source of core fracture is at the FPD connectors with inadequate connector area. Recommendations for connector dimensions are 16 mm2 for lithium disilicate and 9 mm2 for zirconia. Connector fractures originate from veneering porcelain in sharply contoured gingival embrasures where tensile stresses concentrate (Figure 4).21
Polishing and Wear Properties
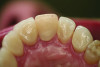
Ceramic hardness ranges from 481 Hv to 647 Hv for veneering porcelain to 1,354 Hv to 1,378 Hv for zirconia.22,23 Since enamel has a hardness of 300 Hv to 500 Hv,24 concerns have been raised that ceramic restorations will cause destructive wear to opposing teeth. Studies measuring the wear of enamel opposing zirconia and lithium disilicate, however, have proven that these high-strength ceramics produce less opposing enamel wear than veneering ceramics or enamel itself.25-28 For example, in-vitro volumetric enamel wear from 400,000 chewing cycles measured 0.33 mm3 against zirconia, 0.36 mm3 against lithium disilicate, 2.15 mm3 against veneering porcelain, and 0.45 mm3 against enamel.26 When veneering porcelains are worn against enamel, the porcelain surface becomes rough from microfractures of the material. The rough surface of the porcelain is abrasive to the enamel and results in opposing enamel wear (Figure 5 and Figure 6).29 High-strength ceramics do not fracture when worn against enamel; therefore, their surface remains smooth and wear-friendly to opposing enamel. Additionally, high-strength ceramics experience very little wear on their own surface. The recent trend in full-contour monolithic lithium-disilicate and zirconia crowns is partially justified by the wear-compatibility between these ceramics and opposing enamel.
To maintain the smooth surface of ceramics after occlusal adjustments, it is important to polish the surface of the zirconia or lithium disilicate. There is significantly less opposing enamel wear when ceramics are polished following grinding than with grinding alone.26 Studies have also compared opposing enamel wear after polishing and glazing zirconia. Glazing zirconia produces a 30-µm– to 50-µm–thick, relatively soft layer of glassy glaze.30 During function, this layer of glaze quickly wears away and the roughened glaze layer causes wear of opposing enamel.27 Clinically, it is recommended to polish ceramic restorations with a heatless alumina stone followed by a silica, silicon carbide, or diamond impregnated rubber polisher.31
Cementation Methods
Ceramic prostheses can be cemented to a tooth preparation either through traditional cementing or adhesive bonding. Traditional cementing relies on micromechanical retention, whereas adhesive bonding utilizes chemical and micromechanical retention (Figure 7). Traditional cementation can be accomplished with glass-ionomer cement (GIC), resin-modified glass-ionomer cement (RMGIC), or zinc-phosphate cement. Adhesive bonding implies that an adhesive bonding agent is applied to the tooth surface, a coupling agent (eg, silane) is applied to the ceramic, and the prosthesis is bonded with a resin cement. The decision to cement or bond a restoration is based on several clinical factors such as: the type of restoring ceramic, the substrate (enamel or dentin), the retentiveness of the preparation, and the ability to isolate the tooth.
When choosing to bond an all-ceramic restoration, the protocol differs for the type of ceramic material. To chemically bond to feldspathic porcelain and reinforced glass ceramics, hydrofluoric acid (Figure 8) and a silane coupling agent (Figure 9) are used. Etchant concentration and etch time vary depending on the ceramic substrate.32 Silane is a coupling molecule that bonds on one end to silica in glass and bonds to the organic matrix of the resin cement on the other end (Figure 10, element B). Silane is applied to the etched intaglio surface of the ceramic crown and bonds the porcelain or glass ceramic to the resin cement. Porcelain and glass ceramics are not alumina air-abraded as this will decrease the strength of the ceramic.33 (The cited abstract is a project completed in the authors’ laboratory, which showed a decrease in flexural strength of lithium disilicate after air abrasion.) Bonding to zirconia or alumina cannot be accomplished with silane alone because there is not enough silica in these materials. Effective strategies for chemically bonding to polycrystalline ceramics include tribochemical silica coating and 10-methacryloyloxydecyl dihydrogen phosphate (MDP) monomer coating.34 The first strategy is to air-abrade the ceramic surface with silica-covered alumina particles, which both roughens the ceramic surface and deposits silica on its surface. The deposited silica can then bond to silane or MDP coupling agents.35 The second strategy is the use of MDP, a bifunctional molecule that bonds to metal oxides (including zirconia) at one end and the organic matrix of resin on the other (Figure 10, element A).36,37
One factor to consider when luting ceramic crowns is the bond strength of the cement. A study by Peutzfeldt compared the shear bond strength of dentin to porcelain (etched and silanated), leucite glass ceramic (etched and silanated), and zirconia (untreated). Cementing porcelain and leucite ceramic with zinc phosphate, glass ionomer, and resin-modified glass ionomer produced lower bond strengths than adhesively bonding them with most resin cements. Conversely, zirconia showed similar bond strength when cemented with RMGIC as with many of the resin cements.38 As mentioned previously, adhesively bonding zirconia with an MDP primer or tribochemical coating will increase its bond strength.36,37 The type of resin cement, such as total-etch (separate etch and primer step), self-etch (separate primer step), or self-adhesive (no etch or primer), can affect the bond strength. Total-etch cements show a higher bond strength than self-etch or self-adhesive resin cements.39 For tooth preparations with less than 3 mm of occlusal height or more than 5 degrees of taper, adhesively bonding zirconia and lithium-disilicate crowns is recommended to achieve sufficient retention.40
Another consideration when selecting a cement is its ability to strengthen the ceramic material. Adhesively bonding feldspathic porcelain to tooth structure increases the fracture strength of the porcelain.41 Heintze demonstrated that leucite and lithium-disilicate crowns adhesively bonded with resin cement had higher fracture strength than those cemented with GIC.42 Another study by Al-Wahadni showed no difference in fracture strength of lithium-disilicate and alumina crowns cemented with GIC or bonded with resin cement.43 Clinical studies have shown no difference in the 8-year success rate of lithium-disilicate crowns cemented with RMGIC or bonded with resin cement.44,45
Conclusion
The choice of ceramic selected for a clinical application is dependent on the required strength and esthetics of the restoration. Broadly, polycrystalline ceramics are stronger and more opaque than glass ceramics and porcelain. The type of ceramic selected will dictate the design of the restoration and the options for luting the restoration. All-ceramic materials should be polished following delivery.
ABOUT THE AUTHORS
Nathaniel C. Lawson, DMD, PhD
Assistant Professor, University of Alabama at Birmingham School of Dentistry, Birmingham, Alabama
John O. Burgess, DDS, MS
Professor and Assistant Dean for Clinical Research, University of Alabama at Birmingham School of Dentistry, Birmingham, Alabama
Queries to the author regarding this course may be submitted to authorqueries@aegiscomm.com.
REFERENCES
1. Apel E, Deubener J, Bernard A, et al. Phenomena and mechanisms of crack propagation in glass-ceramics. J Mech Behav Biomed Mater. 2008;1(4):313-325.
2. Garvie RC, Hannink RH, Pascoe RT. Ceramic steel? Nature. 1975;258:703-704. doi:10.1038/258703a0
3. Kim MJ, Ahn JS, Kim JH, et al. Effects of the sintering conditions of dental zirconia ceramics on the grain size and translucency. J Adv Prosthodont. 2013;5(2):161-166.
4. Heintze SD, Rousson V. Survival of zirconia- and metal-supported fixed dental prostheses: a systematic review. Int J Prosthodont. 2010;23(6):493-502.
5. Anusavice KJ. Standardizing failure, success, and survival decisions in clinical studies of ceramic and metal–ceramic fixed dental prostheses. Dent Mater. 2012;28(1):102-111.
6. Zhao K, Pan Y, Guess PC, et al. Influence of veneer application on fracture behavior of lithium-disilicate–based ceramic crowns. Dent Mater. 2012;28(6):653-660.
7. Aboushelib MN, Feilzer AJ, Kleverlaan CJ. Bridging the gap between clinical failure and laboratory fracture strength tests using a fractographic approach. Dent Mater. 2009;25(3):383-391.
8. Fischer J, Stawarzcyk B, Trottmann A, Hämmerle CH. Impact of thermal misfit on shear strength of veneering ceramic/zirconia composites. Dent Mater. 2009;25(4):419-423.
9. Blatz MB, Bergler M, Ozer F, et al. Bond strength of different veneering ceramics to zirconia and their susceptibility to thermocycling. Am J Dent. 2010;23(4):213-216.
10. Choi EC, Waddell JN, Torr B, Swain MV. Pressed ceramics onto zirconia. Part 1: Comparison of crystalline phases present, adhesion to a zirconia system and flexural strength. Dent Mater. 2011;27(12):1204-1212.
11. Kim HJ, Lim HP, Park YJ, Vang MS. Effect of zirconia surface treatments on the shear bond strength of veneering ceramic. J Prosthet Dent. 2011;105(5):315-322.
12. Kirmali O, Akin H, Ozdemir AK. Shear bond strength of veneering ceramic to zirconia core after different surface treatments. Photomed Laser Surg. 2013;31(6):261-268.
13. Mosharraf R, Rismanchian M, Savabi O, Ashtiani AH. Influence of surface modification techniques on shear bond strength between different zirconia cores and veneering ceramics. J Adv Prosthodont. 2011;3(4):221-228.
14. Guess PC, Bonfante EA, Silva NR, et al. Effect of core design and veneering technique on damage and reliability of Y-TZP-supported crowns. Dent Mater. 2013;29(3):307-316.
15. Rosentritt M, Steiger D, Behr M, et al. Influence of substructure design and spacer settings on the in vitro performance of molar zirconia crowns. J Dent. 2009;37(12):978-983.
16. Fischer J. Strength of zirconia single crowns related to coping design [abstract]. J Dent Res. 2005;84(spec iss A). Abstract 0546.
17. Lin W, Ercoli C, Feng C, Morton D. The effect of core material, veneering porcelain, and fabrication technique on the biaxial flexural strength and weibull analysis of selected dental ceramics. J Prosthodont. 2012;21(5):353-362.
18. Baladhandayutham B, Beck P, Litaker MS, et al. Fracture strength of all-ceramic restorations after fatigue loading [abstract]. J Dent Res. 2012;91(spec iss A):24.
19. Rekow ED, Silva NR, Coelho PG, et al. Performance of dental ceramics: challenges for improvements. J Dent Res. 2011;90(8):937-952.
20. Curtis AR, Wright AJ, Fleming GJ. The influence of surface modification techniques on the performance of a Y-TZP dental ceramic. J Dent. 2006;34(3):195-206.
21. Taskonak B, Yan J, Mecholsky JJ Jr, et al. Fractographic analyses of zirconia-based fixed partial dentures. Dent Mater. 2008;24(8):1077-1082.
22. Pittayachawan P, McDonald A, Young A, Knowles JC. Flexural strength, fatigue life, and stress-induced phase transformation study of Y-TZP dental ceramic. J Biomed Mater Res B Appl Biomater. 2009;88(2):366-377.
23. Shijo Y, Shinya A, Gomi H, et al. Studies on mechanical strength, thermal expansion of layering porcelains to alumina and zirconia ceramic core materials. Dent Mater J. 2009;28(3):352-361.
24. Park S, Quinn JB, Romberg E, Arola D. On the brittleness of enamel and selected dental materials. Dent Mater. 2008;24(11):1477-1485.
25. Rosentritt M, Preis V, Behr M, et al. Two-body wear of dental porcelain and substructure oxide ceramics. Clin Oral Investig. 2012;16(3):935-943.
26. Syklawer SB, Janyavula S, Beck P, et al. Wear of ceramics and enamel in artificial chewing simulator [abstract]. J Dent Res. 2013;92(spec iss A):1902.
27. Janyavula S, Lawson N, Cakir D, et al. The wear of polished and glazed zirconia against enamel. J Prosthet Dent. 2013;109(1):22-29.
28. Kim MJ, Oh SH, Kim JH, et al. Wear evaluation of the human enamel opposing different Y-TZP dental ceramics and other porcelains. J Dent. 2012;40(11):979-988.
29. Oh WS, Delong R, Anusavice KJ. Factors affecting enamel and ceramic wear: a literature review. J Prosthet Dent. 2002:87(4):451-459.
30. Heintze SD, Cavalleri A, Forjanic M, et al. Wear of ceramic and antagonist—a systematic evaluation of influencing factors in vitro. Dent Mater. 2008;24(4):433-449.
31. al-Wahadni A, Martin DM. Glazing and finishing dental porcelain: a literature review. J Can Dent Assoc. 1998;64(8):580-583.
32. Soares CJ, Soares PV, Pereira JC, Fonseca RB. Surface treatment protocols in the cementation process of ceramic and laboratory-processed composite restorations: a literature review. J Esthet Restor Dent. 2005;17(4):224-235.
33. Meenes T, Burgess JO, Cakir D, et al. Abrasion and etching effects on lithium disilicate flexural strength [abstract]. J Dent Res. 2014;93(spec iss A):184572.
34. Mair L, Padipatvuthikul P. Variables related to materials and preparing for bond strength testing irrespective of the test protocol. Dent Mater. 2010;26(2):e17-e23.
35. Atsu SS, Kilicarslan MA, Kucukesmen HC, Aka PS. Effect of zirconium-oxide ceramic surface treatments on the bond strength to adhesive resin. J Prosthet Dent. 2006;95(6):430-436.
36. Attia A, Kern M. Long-term resin bonding to zirconia ceramic with a new universal primer. J Prosthet Dent. 2011;106(5):319-327.
37. Blatz MB, Sadan A, Martin J, Lang B. In vitro evaluation of shear bond strengths of resin to densely-sintered high-purity zirconium-oxide ceramic after long-term storage and thermal cycling. J Prosthet Dent. 2004;91(4):356-362.
38. Peutzfeldt A, Sahafi A, Flury S. Bonding of restorative materials to dentin with various luting agents. Oper Dent. 2011;36(3):266-273.
39. Viotti RG, Kasaz A, Pena CE, et al. Microtensile bond strength of new self-adhesive luting agents and conventional multistep systems. J Prosthet Dent. 2009;102(5):306-312.
40. Powers JM, Farah JW, O’Keefe KL, et al. Guide to all-ceramic bonding. The Dental Advisor. 2009;2:1-12.
41. Jensen ME, Sheth JJ, Tolliver D. Etched-porcelain resin-bonded full-veneer crowns: in vitro fracture resistance. Compendium. 1989;10(6):336-347.
42. Heintze SD, Cavalleri A, Zellweger G, et al. Fracture frequency of all-ceramic crowns during dynamic loading in a chewing simulator using different loading and luting protocols. Dent Mater. 2008;24(10):1352-1361.
43. Al-Wahadni AM, Hussey DL, Grey N, Hatamleh MM. Fracture resistance of aluminium oxide and lithium disilicate-based crowns using different luting cements: an in vitro study. J Contemp Dent Pract. 2009;10(2):51-58.
44. Gehrt M, Wolfart S, Rafai N, et al. Clinical results of lithium-disilicate crowns after up to 9 years of service. Clin Oral Investig. 2013;17(1):275-284.
45. Wolfart S, Eschbach S, Scherrer S, Kern M. Clinical outcome of three-unit lithium-disilicate glass-ceramic fixed dental prostheses: up to 8 years results. Dent Mater. 2009;25(9):e63-e71.