You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
The definition of wellness may vary from one person to another. For some, it is the prime level of functional or metabolic efficiency, usually meaning to be free from illness, injury, or pain. Others interpret it as relating to a healthy balance of the mind, body, and spirit resulting in an overall feeling of well-being. Still, there are those who would say that wellness goes beyond even those parameters to include mental, physical, spiritual, social, occupational, and health conditions, with the overall goal being to seek a more optimal, holistic, and balanced state of health and well-being by utilizing practices from across multiple disciplines.1
In the dental profession, the concept of wellness has gained popularity in recent years as it relates to the concept of dentistry and oral medicine as an integrated part of an overall health plan. As an ever-increasing understanding of the link between oral health and systemic health unfolds, dental practices have begun to focus on explaining to patients why treatments are recommended as much as how the treatment will be done. As such, advances in dental technology have followed this trend with tools designed to detect oral conditions that, if left untreated, could compromise overall systemic health.
Oral Cancer Screening Devices
The incidence of oral and head and neck cancer continues to rise, just as it has over the past 5 years. In previous decades, oral cancer was found mostly in men over the age of 60 with a social history of tobacco and alcohol use. Recently, a new causative factor has taken center stage regarding oral cancer: HPV-16, or the human papilloma virus type 16, the same identified etiology responsible for the majority of cervical cancers in women.2 This new development has altered the concept of risk demographics for oral and head and neck cancers with HPV-related disease found in younger individuals of both genders with no history of deleterious habits.3 Although the incidence of oral and head and neck cancer related to tobacco and alcohol has fallen in recent years, the overall trend for the disease is a steady increase in occurrence, with more cases being attributed to HPV each year. This year, an estimated 42,000 people in the United Sates will be newly diagnosed with oral cancer, or about 115 people per day. Close to 43% of these new cases will not survive 5 years from the time of their diagnosis, as most will not be discovered until the cancer has advanced to a late stage.4 For this reason annual screenings for oral and head and neck cancers are a significant service that dental practices dedicated to patient wellness and systemic health can deliver.
A screening for oral cancer begins well before even looking in the patient’s mouth. Observation for changes in facial symmetry or swelling in the neck begin as soon as the dental professional sees the patient. The patient should be asked questions regarding changes in his or her health. The answers should be documented, and clues that may indicate a problem identified should be noted. Such indications could include the following: a feeling of something caught in the throat, difficulty swallowing, unexplained hoarseness, or a lesion that will not heal. Of course, there is no substitution for the visual and tactile examination that must follow. Simply looking is not enough; palpation is an integral part of a well-executed comprehensive oral cancer screening. But just as the tactile examination offers the practitioner additional information about potential pathological processes, so now do technological advances utilizing the oral mucosa’s natural properties of fluorescence and reflectance aid in identifying intraoral abnormalities.
The purpose of an oral cancer screening adjunctive tool is to aid the practitioner in performing a screening that may detect anomalies in a very early stage of development and that are not yet apparent to the naked eye.5 As such, the use of an oral cancer screening device is appropriate on all occasions, but is in no way meant to replace the standard oral cancer screening. Similarly, use of the adjunctive tool can substantiate suspicions that a lesion might warrant diagnostic tests to determine if an area is indeed cancerous. Some tools are designed to be used intraorally, with the source light very near the suspected lesion. Others have an extraoral light source that is shined upon the tissue in the mouth. One benefit of the intraoral light source is its ability to proximate the beam of light near the tonsils, the base of the tongue, and the oropharynx, where the majority of HPV-related lesions are found. A disposable mirror head, whether incorporated into the design of the adjunctive device or utilized in addition to the light source, is almost always necessary for a light-aided visual examination of structures in the HPV zone near the posterior of the mouth and the superior pharynx.
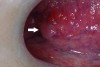
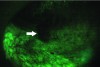
While different devices have varying designs, many take advantage of the oral mucosa’s characteristic of natural fluorescence.6 Fluorescence is the energy expelled by cells after absorbing a high energy input before they return to their normal energy level. The fluorescence characteristics of tissues depend upon their biochemical composition and histomorphological architecture, both of which undergo a change during malignant transformation.7 From the device, a blue or violet light usually at or near a wavelength of 410 nm7 is emitted, which excites natural fluorophores in the mucosa. Through the use of a filter, visualization of changes in the normal fluorescence pattern can be detected. With the use of most devices on the market that use this technology, dysplastic areas of concern appear dark when compared to the surrounding tissue due to the loss of natural fluorescence (Figure 1 and Figure 2). Some devices have additional light phases to supplement the fluorescence examination. Although vascularization is visible under the violet phase, some devices incorporate an amber light to enhance normal tissue’s reflectance properties. Tissue illuminated with amber light, which is absorbed by hemoglobin, can more readily be examined for changes in vascularization patterns deep within the tissue; abnormal tissue has a diffuse system of blood vessels, whereas normal tissue vascularization is clearly defined. Some devices are also equipped with a highly concentrated white light to aid in normal visualization during the conventional screening process.
Regardless of the design, it must be stressed that the oral cancer screening device is meant to be used as an adjunct to the clinical observations, medical history, and patient information gathered and assessed by the practitioner. The device alone does not definitively determine or rule out the presence of oral cancer. It is a screening tool, used to evaluate structures in the head and neck for evidence of cancer before the patient presents with signs or symptoms and while the outcome of treatment, if cancer is found, could be most optimal. Until the tissue has been sampled by biopsy and observed histologically for the presence of malignant cells, a definitive diagnosis of cancer cannot be made.
The same fluorescence technology used to screen for oral cancer can also be used to detect other intraoral anomalies. Areas of inflammation such as those brought about by lichen planus and other lichenoid reactions and trauma are more readily delineated with the use of an adjunctive device. The range of oral and perioral viral, fungal, and bacterial infections can be verified through detection with a fluorescence tool. Squamous papillomas, fibromas, carcinoma in-situ, and mild-to-severe dysplasia are all more readily visible through the use of this chairside technology. When used to its fullest extent, a fluorescence adjunctive device fits well into a dental practice’s wellness plan that goes far beyond simply screening for oral cancer.
Salivary Diagnostic Tests
Beyond the use of oral cancer screening devices, chairside salivary diagnostic tests are another tool in the dental professional’s armamentarium for early detection of disease, as scientific advances in biomarker research have led dentistry and medicine to consider saliva a viable diagnostic medium (Figure 3). These non-invasive tests are often simple to use with accurate results available in a relatively short amount of time. The patient is instructed to swish, and in some instances gargle, with a solution for approximately 30 seconds. The solution is then expectorated into a funneled collection tube, and a cap is secured to protect the specimen during transport. The tube is then sent (typically via FedEx) to a laboratory company for DNA-PCR (polymerase chain reaction) analysis. A final report is then electronically sent to the clinician when ready.8
One available test determines the presence of HPV-16 and HPV-18, another type of human papilloma virus associated with cancer. Because the human papilloma virus cannot be reliably cultured in-vitro, molecular technology is required to detect the presence of the virus. The sample is combined with an extraction buffer to release and denature the target HPV DNA. This denatured DNA can then be combined with specific ribonucleic acid (RNA) probes to create hybrids that can be quantified to detect the presence of the virus. As with an oral cancer screening device, information gathered from the salivary diagnostic test is just one piece of information that must be considered within the context of all findings gathered from the patient’s health history. For example, a positive test for the presence of HPV-16 is not indicative that the patient has oral or cervical cancer. It simply means that the patient is infected with a virus that has been associated with those types of cancer. The patient’s immune system may be able to clear the virus on its own with no presence of disease related to the infection, and even if the patient remains HPV-positive, he or she may never develop cancer as a result of the infection. If, however, the dental professional and the patient are aware of the HPV status, oral cancer screenings can be conducted more often than the recommended annual frequency. The patient should be monitored closely for changes that could indicate an oropharyngeal cancer and be retested annually for a persistent HPV infection.
Other readily available salivary tests can identify the presence and concentration of known periodontal pathogens and determine a patient’s genotype for certain cytokines like interleukin-6 (IL-6) to better assess risk and treatment options for patients with regard to periodontal disease. Again, molecular technologies are employed to detect and quantify the pathogens and cytokines in the sample. According to the Centers for Disease Control and Prevention (CDC), almost half of all Americans have periodontal disease, with nearly 40% having a moderate or severe form of the disease.9 The primary etiology of periodontal disease is long-understood to be bacteria, and more evidence continues to be established about localized and systemic oxidative stress and the effects of inflammation brought about by this common disease process.10 The absence of inflammation and disease is a major tenet of virtually every definition of overall wellness, but moreover, the link between periodontal health and systemic health has been the foundation of understanding how oral health fits into a wellness plan.11 Research has indicated that women with periodontal disease may be at risk for adverse pregnancy outcomes such as premature birth or low-term birth weight babies.12 Additional studies have associated periodontal disease with heart disease,13 diabetes,14 rheumatoid arthritis,15 Alzheimer’s disease,16 obesity,17 and erectile dysfunction in men.18 While not every detail is understood yet about how periodontal disease affects each and every condition mentioned above, oral health influences systemic health on a variety of levels.19 As such, clinicians may be in a better position to treat and manage periodontal disease when information like the kind found in these salivary diagnostic tests is known.
The future of salivary diagnostic testing continues to look promising. According to the American Dental Association (ADA), researchers have reported encouraging findings in validating salivary biomarkers for potential use in the diagnosis of oral cancer and Sjögren’s syndrome. “Lab-on-a-chip” technology may help develop innovative chairside diagnostic tests to identify oral fluid biomarkers associated with good health and other biomarkers that might be associated with oral and/or systemic diseases. Oral fluid diagnostic tests have already been developed to determine HIV status and detect substance abuse. Continued research should enhance point-of-care testing by delivering less invasive and more reliable test methods for the benefit of both the patient and clinician.20
Caries Detection
Another oral manifestation of bacterial infection presents as dental caries. Dental caries is one of the most common diseases in the world today, with approximately 2.43 billion people worldwide experiencing decay in permanent teeth. In the United States, dental caries is the most common chronic childhood disease. If left untreated, dental caries can progress into a dental abscess, which could eventually lead to osteomyelitis, cellulitis, Ludwig’s angina, septicemia, or even death.21,22 Early detection and early intervention, along with prevention, are therefore key elements of any practice focused on the overall well-being of its patients.
Most of the caries detection devices on the market today utilize the alteration of fluorescence, reflectance, electrical conductance, or impedance properties of enamel with demineralization to monitor changes in carious lesions over time. For example, in a laser fluorescence system, changes due to demineralization cause an increase in fluorescence at specific excitation wavelengths. When incident light interacts with tooth substance, it stimulates fluorescent light at longer wavelengths. This generated fluorescent light travels through additional light fibers that act as a filter to eliminate ambient light, and into a microprocessor that analyzes and translates the signal into an acoustic sound and digital display that shows both a real-time and maximum range of demineralization detected during the analysis.
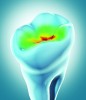
Another caries detection device demonstrates a similar design, but relies on detectable differences of optical behavior inside the tooth related to change in mineralized tooth structure. Infrared and red light-emitting diodes (LEDs) and a fiber optic are used to distribute light to the observed area present at a probe tip. A second fiber optic collects light from the observed area and sends it to a photodetector that measures the returned collected light. This photodetector then transmits the signal to a microprocessor that compares the signal levels with defined parameters. The clinician then observes either the illumination of a green LED to demonstrate healthy tooth structure or a pulsing red LED and a variable-frequency audible buzzer to indicate the presence and intensity of demineralization detected (Figure 4).23
There are various caries detection systems available on the market today. While many utilize similar optical technology, some combine chairside detection with imaging advancements such as tomography to provide detailed information on the infected tooth structure. Regardless of the system adopted based on the personal preference of the clinician, a caries detection system can be an effective diagnostic aid to detect dental caries early and allow for intervention when the preparation of the tooth can be as minimally invasive as possible to eradicate the bacterial invasion and preserve healthy tooth structure.
Conclusion
As society increasingly focuses on health and well-being, many patients are expecting their dentist to be an integral part of their overall wellness plan. Technology adjuncts can enable the practitioner to supply detection services more effectively and at an earlier time to the benefit of the patient’s overall health. Identifying carcinoma in-situ that is difficult to detect with the unaided eye before it becomes stage IV metastatic disease with palpable cervical lymph nodes could make the difference between life and death for a patient. Understanding the HPV status of a patient may allow for closer observation and early intervention should the virus lead to cancer. Even the detection and restoration of carious lesions yet undetectable by the tine of an explorer may decrease the likelihood that the lesion would advance to the point of causing pain and risk systemic health.
In most instances, use of these types of technologies in a dental practice is billable, so the potential exists for a continued revenue stream long after the initial investment has been recovered. Some dental insurances plans will provide compensation for screening and diagnostic tools used. Dental insurance codes such as those listed in Table 1 should be investigated and used when appropriate. Similarly, some medical insurance plans may provide compensation for these types of procedures when they relate to the wellness of the patient.
With patients maintaining healthier lifestyles and living longer, attention to wellness is a service that they are increasingly expecting to receive. Dental practices that implement an oral health/systemic health wellness plan for their patients are in position to be the vanguard of a new age of healthcare where well-being and prevention are the rules and unhealthy issues are detected, managed, and treated at an early stage. Implementation of screening and diagnostic technology can aid in providing early, effective wellness services to patients.
ABOUT THE AUTHOR
Dennis M. Abbott, DDS
Founder and CEO, Dental Oncology Professionals, Garland, Texas; Dental Oncologist, Baylor Charles A. Sammons Cancer Center, Dallas, Texas
Queries to the author regarding this course may be submitted to authorqueries@aegiscomm.com.
REFERENCES
1. Healthy People 2020. Healthy People Web site. http://www.healthypeople.gov/2020/TopicsObjectives2020/pdfs/HP2020_brochure_with_LHI_508.pdf. US Department of Health and Human Services. Published November 2010. Accessed October 10, 2013.
2. Azvolinsky A. HPV linked to increase esophageal cancer risk. Cancer Network Web site. http://www.cancernetwork.com/esophageal-cancer/hpv-linked-increased-esophageal-cancer-risk. Published August 6, 2013. Accessed October 10, 2013.
3. Kreimer AR, Johansson M, Waterboer T, et al. Evaluation of human papillomavirus antibodies and risk of subsequent head and neck cancer. J Clin Oncol. 2013;31(21):2708-2715.
4. Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163-2196.
5. Mendes SF, de Oliveira Ramos G, Rivero ER, et al. Techniques for precancerous lesion diagnosis. J Oncol. 2011. doi:10.1155/2011/326094.
6. Amaechi BT. Emerging technologies for diagnosis of dental caries: the road so far. J Appl Phys. 2009;105(10):102047. doi.org/10.1063/1.3116632.
7. Ingrams DR, Dhingra JK, Roy K, et al. Autofluorescence characteristics of oral mucosa. Head Neck. 1997;19(1):27-32.
8. The OraRisk® HPV Salivary Diagnostic Test. OralDNA Labs Web
site. http://www.oraldna.com/oral-hpv-testing.html. Accessed October 10, 2013.
9. Eke PI, Dye BA, Wei L, et al. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res. 2012;91(10):914-920.
10. Chapple IL, Matthews JB. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontol 2000. 2007;43:160-232.
11. Chapple IL. Reactive oxygen species and antioxidants in inflammatory diseases. J Clin Periodontol. 1997;24(5):287-296.
12. Offenbacher S, Katz V, Fertik G, et al. Periodontal infection as a possible risk factor for preterm low birth weight. J Periodontol. 1996;67(10 suppl):1103-1113.
13. Friedewald VE, Kornman KS, Beck JD, et al. The American Journal of Cardiology and Journal of Periodontology editors’ consensus: periodontitis and atherosclerotic cardiovascular disease. J Periodontol. 2009;80(7):1021-1032.
14. Correa FO, Gonçalves D, Figueredo CM, et al. Effect of periodontal treatment on metabolic control, systemic inflammation and cytokines in patients with type 2 diabetes. J Clin Periodontol. 2010;37(1):53-58.
15. Molitor JA, Alonso A, Wener MH, et al. Moderate to severe adult periodontitis increases risk of rheumatoid arthritis in non-smokers and is associated with elevated ACPA titers: the ARIC study [abstract]. Arthritis Rheum. 2009;60(suppl 10):1160. doi:10.1002/art.26234.
16. Gatz M, Reynolds CA, Finkel, D, et al. Dementia in Swedish twins: predicting incident cases. Behav Genet. 2010;40(6):768-775.
17. Chaffee BW, Weston SJ. Association between chronic periodontal disease and obesity: a systematic review and meta-analysis. J Periodontol. 2010;81(12):1708-1724.
18. Oğuz F, Eltas A, Beytur A, et al. Is there a relationship between chronic periodontitis and erectile dysfunction? J Sex Med. 2013;10(3):838-843.
19. Cullinan MP, Ford PJ, Seymour GJ. Periodontal disease and systemic health: current status. Aust Dent J. 2009;54(suppl 1):S62-S69.
20. Salivary Diagnostics. American Dental Association Web site. http://www.ada.org/2897.aspx. Accessed October 10, 2013.
21. Robertson D, Smith AJ. The microbiology of the acute dental abscess. J Med Microbiol. 2009;58(pt 2):155-162.
22. Li X, Kolltveit KM, Tronstad L, Olsen I. Systemic diseases caused by oral infection. Clin Microbiol Rev. 2000;13(4):547-558.
23. Lussi A, Hibst R, Paulus R. DIAGNOdent: an optical method for caries detection. J Dent Res. 2004;83(spec no C):C80-C83.