You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
Adhesive dentistry has a rich and interesting history. The materials, the rationale for their use, and the successes and failures of various techniques have all contributed to the current science of adhesive dentistry today. In the early 1970s, restorative dentistry was very simple. Clinicians placed either a gold inlay (onlay) or an amalgam in the posterior teeth and a tooth-colored silicate or unfilled metharcylate material in the anterior teeth. The retention of the restoration was purely mechanical. G.V. Black's restorative principles that included undercuts and sharp line angles were easily executed with straight-fissure and inverted-cone-shaped carbide burs. In the anterior teeth a dovetail on the lingual portion of the tooth helped retain larger tooth-colored restorations.
The marginal leakage of restorative materials was as much a problem then as it is today. In 1952, Nelson et al discussed the effect of thermal changes in the oral cavity on the volumetric variations of acrylic filling materials, which resulted in the development of a space between the tooth surface and the filling material. This spacing allowed for a constant fluid exchange to occur between the two surfaces, resulting in secondary decay.1 In 1954, Dr. Ernest Rose spoke of the need to find a way to adhere plastic filling material to tooth structure to eradicate the leakage problem. He presented a study that searched for a material that could sustain adhesion to tooth structure after prolonged immersion in water. After 5,500 tests of various materials, no such solution was found.2 Dr. Michael Buonocore focused on altering the enamel surface to obtain a bond with filling material, and in 1955 he described using 85% phosphoric acid to alter the enamel surface, which resulted in the adherence of an acrylic resin for 1,070 hours submerged under water before detaching.3,4 The idea came from his observation of how phosphoric acid had been used to treat metal surfaces prior to painting to increase the adhesion. His paper3 is the 17th most cited manuscript published in the Journal of Dental Research since 1919, and he is considered the "father of adhesive dentistry."5
However, the first adhesive dental material was actually developed by a Swiss chemist, Oskar Hagger, who in 1949, while working for the DeTrey/Amalgamated Dental Company, introduced a dentin bonding product called Sevriton Cavity Seal.5,6 This product contained an adhesive called glycerolphosphoric acid dimethacrylate, which claimed to penetrate the dentin surface. The penetration of this monomer into the dentin actually formed what is referred to today as a hybrid layer.5 For historical purposes, adhesive dentistry was first started by Oskar Hagger and it was the dentin, not the enamel, that was the initial substrate for bonding.
This article will review the development of bonding materials and techniques. There are certain steps from different bonding approaches that have helped create a new technique that combines immediate dentin sealing with selective acid etching.
1950s: Adhesive Dentistry's Early Years
While Buonocore's seminal paper on adhesion3 in 1955 did not clarify the exact mechanism, it did state that an increase in adhesion of acrylic filling materials to enamel surfaces occurs after 85% phosphoric acid treatment for 30 seconds is employed. He offered a number of possibilities, including: the acid caused an alteration of the organic framework of the enamel; there was an increase in surface area due to the acid-etching action; the acid removed old unreactive enamel; or highly polar phosphate groups remained after the acid removal. It would not be until the late 1960s that the exact mechanism would be published.
In 1956, Bounocore, Brudevold, and Wileman7 evaluated the bond strength of Sevriton Cavity Seal and Sevriton. The cavity sealer, which contained the glycerphosphoric acid dimethacrylate monomer, was applied to the prepared tooth, followed by the resin material. This monomer had a pH of 2.5 and the authors felt that the acidity caused some dissolution of the dentin surface and enlargement of the tubules. This would allow for penetration of the resin into the dentin causing mechanical retention. They then applied the same principle for other standard acrylic resins. Using a drop of 7% hydrochloric acid, the dentin surface was etched for 1 minute, followed by the application of a standard acrylic resin filling material. The adhesive properties of a standard acrylic resin were not increased after the acid treatment. However, the adhesive properties of the Sevriton material doubled. Upon further histologic investigation using staining techniques, they suggested that a chemical reaction might occur between the dentin surface and the monomer.
They concluded that the possibility of bonding resin filling materials to tooth structure was realistic and, furthermore, that it could bond to dentin. They also suggested that a certain chemical structure was necessary for this adhesion to occur. The idea of treating or conditioning the enamel and dentin surface to enhance bond strengths was conceived. Although there was an increase in the bond strength, the immersion in water greatly reduced this bond.8 Water was, and still is today, the greatest deterrent of successful adhesive bonding.
Dr. Robert Purrman and his partner, Eric Schmidt, created the first low-viscosity resin, which was based on their 1958 patented polymerization of aziridine compounds. This led to the manufacturing of impression material.9 The resin, called P-Cadurit, was not hydrolytically stable, nor was it commercially successful.
1960s: 1st Generation of Adhesives
It would be years later that a dentist working at the National Bureau of Standards in Washington, DC, R.L. Bowen, realized the limitations of methylmethacrylate filling material in sustaining a bond with tooth structure when subjected to water and saliva. In his 1965 paper10 Bowen described studies conducted by Schouboe et al11 that showed various bonding materials had good adhesion until they were submerged in water or saliva, and then adhesion was lost. He focused his research towards bonding mechanisms that could couple the filling material and tooth structure. He developed a coupling epoxy resin agent, N-phenylglycine glycidyl methacrylate (NPG-GMA), which was a bifunctional molecule.8,12 A bifunctional molecule has the ability to bond to the dentin on one end and composite resin material on the opposite end.8 Although the bond strength was very low (1 MPa to 3 MPa), it was considered the first generation of bonding adhesives.8,12
The first commercially available product to use NPG-GMA monomer was Cervident (S.S. White). To condition the prepared tooth, a solution of 50% phosphoric acid with a 7% zinc-oxide buffer was applied and then rinsed with water for 1 minute, then dried for 1 minute. The NPG-GMA monomer was then applied, which was capable of chelating with the calcium ion on the etched surface of the tooth and then bonding to the restorative resin.13 The bond strength was very low (2 MPa to 3 MPa) and the components were unstable during storage.14 The material had poor clinical results when used as a restorative for abated cervical lesions without mechanical retention.15 In some clinical trials, a 50% failure rate was reported.16 Wolinsky et al used nuclear magnetic resonance to determine ionic bond formations of several adhesives, including the NPG-GMA monomer with hydroxyapatite, and found very little.17
In a 1963 publication,18 Buonocore described the strong adhesion of methylmethacrylate resins to extremely dry teeth, stating that as long as teeth stay dry the adhesion will remain firm; however, once submerged in water, the adhesion is rapidly lost. He compared it to how acrylic resins stick to glass dappen dishes but once they are submerged into water the acrylic floats off the glass. He concluded that water has a greater affinity for enamel, or in this case, glass, than it does for acrylic resin.18
Understanding that moisture was still a problem and that it inhibited the polymerization process,19 in 1962 Bowen created a dimethacrylate by attaching a methylmethacrylate to the end groups of the epoxy resin. This resulted in a new resin called bisphenol A glycidyl methacrylate, or Bis-GMA.19
Bounocore's vision was to successfully prevent caries in posterior teeth by sealing pits and fissures with a bonded resin material.20 The first publication on the use of phosphoric acid to etch the enamel and apply Bis-GMA resin as a pit-and-fissure sealant was by Cueto and Bounocore in 1965.21 Gwinnett and Bounocore followed with a similar report on adhesion in the same year.22 Then in 1967, Cueto and Bounocore generated an additional report in which they eventually used a 50% phosphoric acid buffered with 7% zinc-oxide etchant.23 After 1 year they reported a reduction in caries by 85% with a 71% retention.
The first commercial product to use a Bis-GMA resin was Addent 12™ (3M Dental) and later Addent 35™ (intended for posterior teeth). The Bis-GMA resin provided the Addent material with less polymerization shrinkage and greater color stability. The system was comprised of a powder and a liquid that had a chemical cure.24 One of the techniques used for application was to wet a small brush with the liquid, pick up a small amount of the powder, carry it to the tooth cavity preparation, and wait for the chemical polymerization to complete. This was referred to as the "brush-on" technique.25
In 1966, Langeland et al26 compared the pulp reaction to Addent with silicate cements and cold-curing filling materials. They concluded that the Addent restorative possessed clinical promise for proximal and gingival restorations. The use of an experimental liner reduced the pulp reactions that were experienced in the original Addent formulation. The use of the liner, which was a film-forming synthetic vinyl copolymer dissolved in a volatile solvent, reduced the pulp reaction to levels similar to but not as severe as the cold-curing plastic materials.
The mechanism of acid-etch enhanced adhesion was not published until 1968,27 when Buonocore, Matsui, and Gwinnett discussed the effect of phosphoric acid conditioning, which produced "prism-like" tags of resin materials that penetrated enamel surfaces. These resin tags were not seen in unconditioned enamel. The effect of phosphoric acid on enamel resulting in increased adhesion was now part of the dental literature, but it would be many years later that this principle would be widely accepted.
Dr. Harold R. Stanley published a key article in 197128 that described various controversial clinical problems and provided pertinent biologic knowledge to resolve these issues. One problem discussed was the fact that phosphoric acid was used in silicate cements and zinc phosphate cements. Stanley described how the harmful effects of phosphoric acid seepage into dentinal tubules should be prevented by using a zinc oxide and eugenol cement for all fixed restorations for several months while waiting for the formation of reparative dentin to occur and sealing off the patent tubules. Another technique he recommended was to use calcium hydroxide and cavity varnish to prevent phosphoric acid from either silicates or cements from penetrating dentinal tubules. If the patient did not report a "sting" then the lining was successful in sealing the tubules.28
The first paste-to-paste system to use Bis-GMA resin was Adaptic® (Johnson and Johnson), which was introduced in 1969. Because of its ease of use and initial esthetic results it dominated market share for some time. The kit came with a mixing pad, double-ended plastic disposable spatulas, one 14-gram tub of catalyst paste, and one 12-gram tub of universal paste. There was only the one shade.
Lee and Swartz used a scanning electron microscope (SEM) technique to study the marginal adaptation of four commercial composites: Addent 12, Addent 35, Dakor (L.D. Caulk Co.), and Adaptic. They wanted to compare these materials to clinically established restorative materials: silicate, amalgam, and methylmethacrylate. In conjunction with radioisotope leakage testing, the Adaptic material was found to have the closest cavity and marginal adaptation and least leakage compared with any of the other materials.29
Tobias et al published their study in 197330 on the effects of composite material on dental pulp, specifically resin-bonded quartz composite Adaptic. They found that the restorative material lacked any ingredients that were toxic to the pulp. However, if the material was placed into an unlined cavity then a significant pulpal response would occur. Therefore, this composite material, like so many other similar materials, should be placed only after the cavity has been lined with a protective barrier. The liners used in the study on 96 dog teeth were calcium hydroxide and cyanoacrylate.30
1970s: Breakthroughs
A major breakthrough in adhesive dentistry was the introduction of the pit-and-fissure photo-initiated composite system called Nuva-Seal® (L.D. Caulk Co.). It had a curing initiator and an ultraviolet light source, the Caulk Nuva Lite.20 The procedure for applying this material consisted of cleaning the occlusal surfaces with a nonfluoridated prophy paste and then washing thoroughly. The teeth were dried and isolated with cotton rolls and etched with 50% phosphoric acid for 1 minute. The surfaces were then rinsed thoroughly with water, dried, and isolated again. The sealant was applied and then exposed to the ultraviolet light for 30 seconds.
Although acid etching was used in conjunction with pit-and-fissure sealants, its routine use in restorative dentistry was still not fully accepted. In 1973, Vojinovic et al31 published their study on treating cavities, including the exposed dentin, with citric acid prior to placement of the filling. They also wanted to see if this treatment would reduce bacteria growth between the filling and cavity walls or increase the occurrence of bacteria. It was understood that enamel etching improved bonding to enamel but other researchers suggested that pretreatment of dentin with acids might further improve the bonding of resin.32,33 Twenty-three intact pairs of contra-lateral teeth were used in the study, and after 3 to 4 weeks they not only found a thick layer of bacteria under all the fillings but also found bacteria in the dentinal tubules of the teeth that had been treated with acid. The pulpal reactions were also stronger in the acid-treated teeth. The authors did not recommend utilizing this treatment before inserting a resin material.
In 1974, the first International Symposium on "The Acid Etch Technique" was held in St. Moritz, Switzerland, which hosted researchers and clinicians from all over the world who were working with enamel etching. The symposium was sponsored by the 3M Company, which afterwards provided a publication (The Acid Etch Technique) of the research papers presented as well as commentary on the techniques by other authors. This was a significant contribution in the development of adhesive bonding. Conditioning the enamel, and only the enamel, was now an accepted technique. Over the years, changes would be made as to the concentration of the acid and etching times.
The following year Stanley et al published their study that evaluated human pulp response to pretreatment of dentin with phosphoric and citric acid prior to placement of newer composite materials (HL-72 and Enamelite, Lee Pharmaceuticals, Inc.). They stated that acid conditioning of the enamel had been reported, evaluated, and accepted as a means of improving the bonding mechanism of resin restorative materials. However, they also stated that there was no current proof claiming that acid pretreatment of dentin improved bonding. At the time, some researchers advocated the use of acid pretreatment as a means of cleansing the cavity of debris, blood, and denatured collagen.28,34
The Stanley group placed 150 restorations in teeth that were scheduled to be extracted. The restorations consisted of different combinations of acid-pretreated preparations. The cavity preparations were placed as deep as possible without causing a pulp exposure. Histological studies were performed at different time intervals after extractions were completed. They concluded that all the products in the study caused pulp irritation and, in some cases, abscess formation. Their recommendation was not to use these products unless a cavity liner, such as calcium hydroxide, was applied before conditioning. This was especially necessary when less than 1 mm of dentin remained between the restoration and the pulp tissue.35
In the 1977 edition of Current Therapy in Dentistry, Vol 6, Berry and Laswell described the status of enamel conditioning with composite resins.36 The acid-etch technique they described at the time entailed using 37% to 50% phosphoric acid applied for 1 minute. They also discussed cavity preparation in terms of retention design. They recommended that there remained a need for the G.V. Black preparation parameters, and there was discussion related to the etched enamel providing a secondary retentive system. It was suggested that Class IV lesions and enamel defects could be restored without the use of macro-retention or pins but Class II and Class V should still have micro-retention provided by a 1/4-round bur. In regards to the use of an acid etchant, they described the importance of understanding the potential hazard to the pulp and that a layer of calcium hydroxide should coat all exposed dentin.
Research published between 1971 and 1977 (mostly by Brannstrom) suggested that bacterial irritation was the main cause of pulpal damage.37-43 Brannstrom and Nordenvall44 concluded that the contraction gap between the composite restoration and enamel provided the avenue for the bacterial invasion and this was a potential risk for the development of secondary caries and pulpal irritation in unlined cavity preparations. To reduce this risk it had earlier been suggested by Johnsson and Brannstrom45 that a lining material should cover all surfaces of the cavity preparation except the lateral walls of the enamel. The technique involved placing a two-part liner that consisted of shellac dissolved in alcohol and benzalkonium chloride followed by the application of a polystyrene and copaiba balsam dissolved in ethyl alcohol prior to acid etching the lateral and margin enamel walls.
In their study, Brannstrom and Nordenvall concluded that there was no advantage to etching the dentinal wall of the cavity preparation. An improved cavo-surface marginal seal could be accomplished by only applying an acid gel to the enamel walls and the enamel adjacent to the margins.44
The first system that was considered a new generation of bonding adhesives (2nd generation) was ClearfilTM Bond System F (Kuraray Dental) in Japan in 1977.46 This system had a different approach in that a negatively charged phosphate-ester material (phenyl-P and HEMA in ethanol) interacted with the positively charged calcium in the smear layer.47 This was the first system that chemically adhered to dentin and enamel.48
1980s: New Techniques Emerge
In the early 1980s, the prevailing attitude towards bonding to dentin was that the bond strength was poor and possible sensitivity problems could occur if the dentin was acid etched.49 Fusayama advocated the use of acid etching the dentin to protect the pulp by tightening the seal at the tubular aperture and improving the overall adhesion.48,49 This information was not accepted in Europe or the United States.50 In fact, while this "total-etch" concept became quite popular in Japan it would take many years before it expanded to the United States.51,52 When the 2nd generation of dentin adhesives was introduced, there was a small increase in the bond strengths. Other phosphate-ester bonding systems were introduced at this time. Although the smear layer was left intact, treatment of the dentin surfaces involved the use of a cleansing agent. Most of the bond strength came from the bonding agent adhering to the smear layer.8 The bond strengths of this generation of adhesives ranged between 4.5 MPa and 6 MPa.53,54
The next generation (3rd) of adhesives also brought new techniques. These adhesives focused attention on the prepared dentin surface and the smear layer. The smear layer was either modified or removed without disturbing the smear plugs.55 The bonding systems were mostly comprised of three parts: conditioner, primer, and adhesive. The conditioner consisted of a weak organic acid (maleic acid) or a low concentration of phosphoric acid that provided a partial dissolution of the smear layer.50 Depending on the type of conditioner used, the permeability of the dentin increased.56 Demineralization of the peritubular and intertubular dentin exposed some of the collagen fibers.8 The bonding steps were more technique-sensitive and time-consuming than previous systems and reached between 8 MPa and 15 MPa in bond strength.55
In 1987, the American Dental Association granted the first "full acceptance" of a dentin adhesive,46 which conditioned the smear layer with 2.5% maleic acid, 55% 2-hydroxyethyl methacrylate (HEMA), and a small amount of methacrylic acid. The approach to smear layer conditioning was a combination of the more aggressive Japanese style and the more conservative European and American approach.46
In 1980, Fusayama advocated the etching of dentin to improve overall adhesion.49 Two years later, Nakabyashi et al published their report on the safety and improved adhesion of exposing the dentinal tubules through acid etching. He used a 10% citric acid/3% ferric chloride solution to etch the dentin and applied monomers with hydrophobic and hydrophilic groups. This combination resulted in a bond strength of 18 MPa.57 In 1985, Nakabyashi described the adhesion between the monomer and dentin as not a chemical reaction but as inter-penetration of the monomer into the hard tissue. After removing the smear layer with aqueous 10% citric acid and 3% ferric chloride solution prior to adhesion the infiltrated methacrylates polymerized. This resin-reinforced layer was described as having good resistance against acid with a bond strength of 18 MPa to dentin and 14 MPa to enamel. Interestingly, when he used phosphoric acid the bond strength dropped to 6 MPa, an indication that this acid was too strong. He described the resin-reinforced dentin and enamel as a hybrid of natural tissue and artificial material and concluded that this layer would deter secondary caries after restoration.58 This technique was referred to as "total etch."49
1990s: Adhesion Simplified
The total-etch technique was adopted but still presented postoperative sensitivity problems. Patient demand for "tooth-colored" or composite filling increased, and clinicians were presented with postoperative complaints ranging from cold sensitivity to discomfort with chewing. Some clinicians returned to using amalgam. The total-etch procedure consisted of applying phosphoric acid ranging in concentration of 37% to 40% for 15 to 60 seconds. The etchant was rinsed and the dentin was dried, followed by the application of a primer and adhesive. Kanca discovered that by keeping the dentin moist after the etching step the bond strength improved while the postoperative sensitivity decreased.59 Gwinnett60 and Sugizaki61 published independent studies with similar conclusions at the same time. This technique was referred to as "wet bonding"62 and gave rise to the 4th generation of dental adhesives.
A problem with the wet-bonding technique was that it was difficult to quantify the amount of "wetness" left on the etched dentin surface. After the etching step was completed a demineralized collagen meshwork remained. One of the roles of the primer was to push the water out from the collagen meshwork; however, if some of the water remained then phase changes occurred with the adhesive component, resulting in lower bond strengths and postoperative sensitivity.62,63 This made the sensitivity of the technique highly precise. Manufacturers decided to simplify the technique by combining some of the steps, which gave rise to the 5th generation of adhesives. Even though the bonding materials contributed to improved bond strengths, deviating from the precise technique steps posed clinical postoperative problems for the patient.64 Perdigão et al65 found in their study that the clinical technique was more responsible for postoperative sensitivity than the adhesive materials themselves.
The industry went in two different directions with the introduction of the new 5th generation of dentin adhesives. One direction was to combine the primer and the adhesive and apply them after acid etching with phosphoric acid. Even though these bonding adhesives still required a separate etching step they were marketed as "one-step" systems.50 Studies showed that these systems had good bond-strength values that were created by resin tags, adhesive lateral branches, necessary in the formation of a hybrid layer.8,66
The other direction was to combine the etchant with the primer as the first step followed by application of the adhesive in the second step. This process was referred to as "self-etching." The advantage of this method was the removal of the separate acid-etching step and the elimination of moisture remaining on the dentin surface. The self-etch primers could be used on dry dentin.62
Both variations of the 5th generation of bonding adhesives reduced the steps and technique-sensitivity of adhesion dentistry. However, studies suggested the previous generation of adhesives had better performance.67-70 A major concern was that the pH of the phosphoric acid produced a more aggressive etch pattern on the enamel than the less aggressive (higher pH) self-etching primers.8,71 In fact, clinical and laboratory leakage test results confirmed that the "one-bottle" systems showed that the enamel seal was actually better than the "self-etching" primer version.8,71,72
Sealing the Dentin
In 1992, Pashley et al first proposed sealing the exposed dentinal tubules prior to impressing a prepared tooth to receive an indirect restoration due to the microleakage of the provisional restoration, which resulted in bacterial contamination.73 This technique, which has been referred to as prehybridization,74 the dual-bonding technique,75 resin coating,76 and immediate dentin sealing,77 decreased the chances of bacterial contamination stemming from the poor sealing provided by provisional restorations.73,77-80 Some studies showed an increase in the bond strength due to an additional layer of adhesive that was applied during the insertion phase of an indirect restoration81-84 and a cushioning of the occlusal load resulting from an increase in the adhesive thickness.81,85,86
The technique used the "total-etch-wet-bonding" approach with either a two- or three-bottle adhesive system. The enamel and dentin were etched with phosphoric acid, rinsed with water, and dried, leaving a moist surface. The primer was applied, followed by the adhesive, or a combined primer/adhesive was used and then light-polymerized. The oxygen-inhibited layer that remained over the surface was removed by covering the adhesive layer with a water-soluble gel, then further light-polymerized. This step was critical prior to taking an impression for an indirect restoration. Elimination of the oxygen-inhibited layer prevented interference with the setting of the impression material and adherence of the provisional restorative.87
2000s: Self-Etching Popularized
Characterized by the elimination of phosphoric acid, the 6th generation of adhesives was introduced in the early 2000s. Phosphoric acid was replaced with an acidic primer and the systems were referred to as "self-etching" adhesives.88 The technique sensitivity of the two previous generations led manufacturers to reduce postoperative sensitivity by removing the phosphoric acid etching from the process, which in turn, popularized self-etching adhesives.89,90 There were two types of 6th generation adhesives: one type divided the primer and the adhesive into separate steps and was either dual-cure or light-polyermized88; the other type, which was light-polymerized only, contained the etch, primer, and adhesive all in one bottle.
To make adhesive dentistry even less technique-sensitive and more convenient, the industry moved to place all the bonding steps into one bottle. This gave rise to the 7th generation of bonding agents. Launch dates for these all-in-one bottle systems were 2002-2003.50 Although these 7th generation adhesive systems were easier to use, the results did not match the level of the 4th generation systems,91 which are still considered the gold standard.5,92,93
Instead of removing the smear layer, the 6th and 7th generation bonding agents incorporate the smear layer into the adhesive process.94 The smear layer and underlying dentin and enamel are demineralized by the acidic primer. The bonding premise is basically the same among self-etching adhesives but they differ in other respects such as the type of acidic monomer, the water content, and the acidity.95 The pH of the monomer determines the depth of the demineralization and the thickness of the hybrid layer.96 However, the pH of the monomers is still less acidic than phosphoric acid, which results in less demineralization in the enamel.97,98 These adhesives can be divided into three different categories based on their acidity: the mild acidic adhesives have a pH above 2; the adhesives that are considered strong have a pH below 1; and the intermediate adhesives have a pH of between 1 and 2.99 Studies have shown the 7th generation systems to have greater microleakage than previous generations, especially in areas of uncut enamel.100-102 The integrity of the enamel bond is the first line of defense to microleakage in the success of a restoration. There is some concern that the industry's direction has been focused more on simplifying and strengthening dentin bond systems while sacrificing the strength of enamel bond.103
Suggestions to modify the steps for 7th generation adhesives include the use of phosphoric acid on the enamel prior to application of the one-step adhesive.95,104-106 This modification of acid etching only the enamel to increase the porosity is referred to as selective etching105 (Figure 1). However, when using this technique with some adhesive systems, the dentin bond strength can be compromised if the etchant is inadvertently applied to the dentin due to demineralization.107,108 Torii et al109 investigated what effect etching the enamel and dentin prior to application of a self-etching adhesive would have on the microtensile bond strength and found that it was increased on the enamel but was decreased with the dentin. Another potential problem with dentin etching is that the self-etching primer may not infiltrate as deep as the phosphoric acid etchant, resulting in voids, which in turn can result in postoperative complications such as collagenous degradation, postoperative sensitivity, and microleakage.
Selective etching would include the application of 37% phosphoric acid to the enamel periphery for 15 seconds. After thoroughly rinsing with water, the self-etching adhesive would then be applied to both the dentin and the enamel and light-polymerized in the usual manner. This modified technique has been shown to increase the enamel bond strength for mild self-etching adhesives.110 Rotta et al95 found the bond strength of a low-pH self-etching adhesive was improved by pretreating the enamel with phosphoric acid.
Immediate Dentin Sealing/Selective Etching Combination
As previously mentioned, immediate dentin sealing using a self-etch system was originally discussed by Pashley, and then by others who focused on total-etch systems.74-77 A possible modification would be to combine segments of the immediate dentin sealing process with the selective etching technique for indirect restorations. This combination of generational bonding steps would involve the use of a self-etching adhesive to seal the dentin immediately after the tooth preparation phase is completed. Elimination of the oxygen-inhibited layer would be necessary to prevent contamination of the impression material.111 Once the dentin is sealed, the enamel would be re-prepared with either a carbide or diamond bur, removing the adhesive-impregnated enamel and exposing freshly cut enamel. The impression (two-appointment) or digital scanning (chairside one-appointment) would then be completed. A provisional restoration would be placed in the two-appointment scenario. With the dentin sealed, the chances of postoperative sensitivity are very low.73
The insertion step in a two-appointment scenario would begin with the removal of the provisional restoration and the use of airborne-particle abrasion to remove any biofilm present on the prepared surfaces. This step would not be necessary for the chairside fabrication scenario. In both situations, after the try-in step is completed, 37% phosphoric acid is applied to the entire preparation surface to cleanse the dentin of salivary proteins and etch the enamel. The deeper porosities created in the enamel by the etchant would allow for deeper resin tag formation. The previous application of self-etching adhesive would protect the dentinal surface from demineralization by the etchant and only the cut enamel would be affected.
Case Study
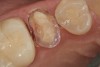
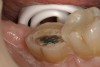
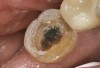
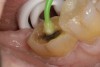
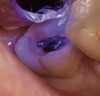
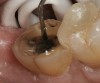
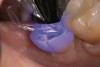
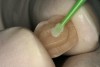
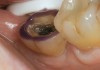
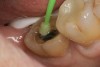
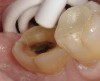
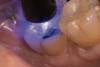
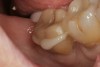
A 70-year-old patient presented with symptoms of a cracked tooth scenario, with intermittent pain upon chewing and thermal sensitivity. The diagnosis was supported through various tests, which determined the lower right second molar had an incomplete fracture that extended from the distal marginal ridge to the mesial marginal ridge. After treatment options were presented, an all-ceramic restoration was decided upon. The tooth was prepared for a nonretentive, adhesively retained all-ceramic restoration ("table top" preparation) (Figure 2 and Figure 3). After the preparation was completed a mild self-etching adhesive was applied to the dentin according to the manufacturer's instruction (Figure 4). There was little concern, if any, of the adhesive reaching the band of exposed enamel. The adhesive was light-polymerized for 5 seconds to secure the layer to the dentin (Figure 5). To eliminate the oxygen-inhibited layer a water-soluble gel was applied to the adhesive and further light-polymerization was completed (Figure 6 and Figure 7). For direct digital scanning cases in which a powdering technique is employed for image capture, it may be more difficult to remove the powder after scanning if the oxygen-inhibited layer is still present.
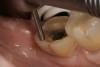
The next step was to re-prepare the enamel band on the outer perimeter of the tooth with either a carbide finishing bur or a diamond end-cutting bur (Figure 8). This removed any adhesive that inadvertently impregnated the enamel. At this point, the tooth was digitally scanned; if a two-appointment scenario had been utilized then the impression would have been taken and a provisional restoration placed.
The insertion step is slightly different depending on whether the restoration is completed chairside while the patient waits (one-appointment) or an impression is sent to the laboratory (two-appointment). As previously mentioned, for the two-appointment scenario, the provisional restoration is removed and the prepared tooth surface is airborne-particle abraded using 50-µm aluminum oxide at 35 psi. This step is not used in the chairside one-appointment scenario. From this point forward all the steps are the same for both situations.
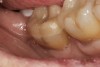
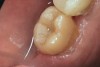
The fit of the restoration was tried in and confirmed. The bonding surface of the ceramic restoration can be prepared by either using a conventional hydrofluoric acid conditioning followed by the application of a silane product, or an alternative method, which was used in this case, whereby phosphoric acid is applied to cleanse and hydrogenate the ceramic surface, followed by the application of a non-etching ceramic primer (Figure 9 and Figure 10). The tooth was then prepared by etching the entire prepared surface with phosphoric acid for 15 to 20 seconds then rinsed with water and dried (Figure 11). Only the enamel at this point was exposed to the phosphoric acid because the immediate dentin sealing step was previously completed. This selective etching step was accomplished without being concerned about etchant exposure on the dentin.
A mild self-etch adhesive was then applied to the entire prepared surface of the tooth. When selecting a self-etching adhesive for this step, the film thickness is critical for complete seating of the restoration. There are several self-etching adhesive systems that have a film thickness of 10 µm or less. Once the adhesive was applied to the tooth and air-thinned (Figure 12 and Figure 13), it was light-polymerized (Figure 14). A dual-cure resin cement by the same manufacturer was mixed and applied to the bonding surface of the restoration, which, in turn, was placed on the tooth (Figure 15). Using a metal instrument, the restoration was held in place while the buccal and lingual surfaces were light-polymerized for 5 seconds each on each side to tack in place. Dental floss was passed through the mesial side to gain interproximal access after the final cure. Light-polymerization was continued as per the manufacturer's recommendation. The excess cement was then removed and a final check of the occlusion was performed (Figure 16 and Figure 17). Several weeks later, the patient reported that there had not been any postoperative sensitivity since the restoration had been placed.
Conclusion
The history of bonding techniques represents a remarkable era in dentistry. Understanding the physical and chemical components of the various adhesive materials and the interaction with direct and indirect restorative materials and the underlining tooth substrate has been critical to the success of adhesive dentistry. Using parts of different bonding techniques has led to the development of a method to seal the dentin and increase the enamel bond strength. Combining techniques has reduced the chances of postoperative sensitivity as seen with 6th and 7th generation adhesives and has increased the bond strength characterized with 4th generation materials.
References
1. Nelsen RJ, Wolcott RB, Paffenbarger GC. Fluid exchange at the margins of dental restorations. J Am Dent Assoc. 1952;44(3):288-295.
2. Rose EE, Lal J, William -588.
3. Buonocore MG. A simple method of increasing the adhesion of acrylic filling materials to enamel surfaces. J Dent Res. 1955;34(6):849-853.
4. Lopes GC, Thys DG, Klaus P, et al. Enamel acid etching: A review. Compend Contin Educ Dent. 2007;28(1):18-25.
5. Söderholm KJ. Dental adhesives…how it all started and later evolved. J Adhes Dent. 2007;9(Suppl 2):227-230.
6. Mclean JW. Historical overview: the pioneers of enamel and dentin bonding. In: Roulet JF, Degrange M, eds. Adhesion: The Silent Revolution in Dentistry. Chicago, IL: Quintessence Publishing; 2000:13-17.
7. Brudevold F, Bounocore M, Wileman W. A report on a resin composition capable of bonding to human dentin surfaces. J Dent Res. 1956;35(6):846-851.
8. Kugel G, Ferrari M. The science of bonding: from first to sixth generation. J Am Dent Assoc. 2000;131(Suppl):20S-25S.
9. McLean JW. Dental materials developments in the UK: a personal view. J Dent Res. 1996;75(11):1816-1819.
10. Bowen RL. Adhesive bonding of various materials to hard tooth tissues. II. Bonding to dentin promoted by a surface-active comonomer. J Dent Res. 1965;44(5):895-902.
11. Schouboe PJ, Paffenbarger GC, Sweeney WT. Resin cements and posterior-type direct filling resins. J Am Dent Assoc. 1956;52(5):584-600.
12. Setcos JC, Staninec M, Wilson NH. The development of resin-bonding for amalgam restorations. Br Dent J. 1999;186(7):328-332.
13. Jedrychowski JR, Caputo AA, Folinart R. Effects of adhesion promoters on resin-enamel retention. J Dent Res. 1979;58(4):1371-1376.
14. Charlton DG. Dentin bonding: past and present. Gen Dent. 1996;44(6):498-507.
15. Das UM, Suma G. Bonding agents in pit and fissure sealants: A review. Inter J Clin Ped Dent. 2009;2(3):1-6.
16. Harris RK, Phillips RW, Swartz ML. An evaluation of two resin systems for restoration of abraded areas. J Prosthet Dent. 1974;31(5):537-546.
17. Wolinsky LE, Armstrong RW, Seghi RR. The determination of ionic bonding interactions of N-phenyl glycine and N-(2-hydroxy-3-methacryloxypropyl)-N-phenyl glycine as measured by carbon-13 NMR analysis. J Dent Res. 1993;72(1):72-77.
18. Buonocore MG. Principles of adhesive retention and adhesive restorative materials. J Am Dent Assoc. 1963;67:382-391.
19. Söderholm KJ, Mariotti A. BIS-GMA-based resins in dentistry: are they safe? J Am Dent Assoc. 1999;130(2):201-209.
20. Simonsen RJ. Pit and fissure sealant: review of the literature. Pediatr Dent. 2002;24(5):393-414.
21. Cueto EI, Buonocore MG. Adhesive sealing of pits and fissures for caries prevention. J Dent Res. 1965;44:137.
22. Gwinnett AJ, Bounocore MG. Adhesives and caries prevention; a preliminary report. Br Dent J. 1965;119:77-80.
23. Cueto EI, Bounocore MG. Sealing of pits and fissures with an adhesive resin: its use in caries prevention. J Am Dent Assoc. 1967;75(1):121-128.
24. Albers H. Tooth-Colored Restoratives: Principles and Techniques. 9th ed. Ontario, Canada: BC Decker Inc.; 2002:111.
25. Fusayama T, Inoue M, Hirano T, Hosoda H. Crazing of acrylic fillings in relation to various inserting, finishing, and polishing techniques. J Dent Res. 1964;43(2):187-193.
26. Langeland LK, Guttuso J, Jerome DR, Langeland K. Histologic and clinical comparison of Addent with silicate cements and cold-curing materials. J Am Dent Assoc. 1966;72:373-385.
27. Buonocore M, Marsui A, Gwinnett AJ. Penetration of resin dental materials into enamel surfaces with reference to bonding. Arch Oral Biol. 1968;13(1):61-70.
28. Stanley HR. Pulpal response to dental techniques and materials. Dent Clin North Am. 1971;15(1):115-126.
29. Lee HL, Swartz ML. Sealing of developmental pits and fissures. I. In vitro study. J Dent Res. 1971;50(1):133-140.
30. Tobias M, Cataldo E, Shiere FR, Clark RE. Pulp reaction to a resin-bonded quartz composite material. J Dent Res. 1973;52(6):1281-1286.
31. Vojinovic O, Nyborg H, Brannstrom M. Acid treatment of cavities under resin fillings: bacterial growth in dentinal tubules and pulp reactions. J Dent Res. 1973;52(6):1189-1193.
32. Retief DH. The intra-oral factors affecting adhesion. J Dent Assoc S Afr. 1970;25(11):392-399.
33. Glantz PO, Larsson LA. The wettability of composite resinous filling materials. Acta Odontol Scand. 1971;29(5):539-548.
34. Lee HL, Cupples AL, Schubert RJ, Swartz ML. An adhesive dental restorative material. J Dent Res. 1971;50(1):125-132.
35. Stanley HR, Going RE, Chauncey HH. Human pulp response to acid pretreatment of dentin and to composite restoration. J Am Dent Assoc. 1975;91(4):817-825.
36. Goldman HM, Gilmore HW, Irby WB, McDonald RE. Current Therapy in Dentistry. 6th ed. St. Louis, MO: CV Mosby; 1977:197-213.
37. Brannstrom M, Nyborg H. The presence of bacteria in cavities filled with silicate cement and composite resin materials. Sven Tandlak Tidskr. 1971;64(3):149-155.
38. Brannstrom M, Nyborg H. Pulpal reaction to composite resin restorations. J Prosthet Dent. 1972;27(2):181-189.
39. Brannstrom M, Nyborg H. Cavity treatment with a microbicidal fluoride solution: growth of bacteria and effect on pulp. J Prosthet Dent. 1973;30(3):303-310.
40. Brannstrom M, Nyborg H. Bacterial growth and pulpal changes under inlays cemented with zinc phosphate cement and Epoxylite CBA 9080. J Prosthet Dent. 1974;31(5):556-565.
41. Brannstrom M, Nyborg H. Pulpal reaction to polycarboxylate and zinc phosphate cements used with inlays in deep cavity preparations. J Am Dent Assoc. 1977;94(2):308-310.
42. Brannstrom M, Vojinovic O. Response of the dental pulp to invasion of bacteria around three filling materials. ASDC J Dent Child. 1976;43(2):83-89.
43. Qvist V. Pulp reactions in human teeth to tooth colored filling materials. Scand J Dent Res. 1975;83(2):54-66.
44. Brannstrom M, Nordenvall KJ. Bacterial penetration, pulpal reaction and the inner surface of Concise enamel bond. Composite fillings in etched and unetched cavities. J Dent Res. 1978;57(1):3-10.
45. Johnsson G, Brannstrom M. Reinigung und isolierung präparierter flächen (II). Die Quintessenz. 1976;27(4):123-128.
46. Roberson TM, Heymann HO, Swift EJ. Sturdevant's Art and Science of Operative Dentistry. 4th ed. St. Louis, MO: Mosby; 2002:244.
47. Retief DH, Denys FR. Adhesion to enamel and dentin. Am J Dent. 1989;2(Spec No.):133-144.
48. Fusayama T. The problems preventing progress in adhesive restorative dentistry. Av Dent Res. 1988;2(1):158-161.
49. Vaidyanathan TK, Vaidyanathan J. Recent advances in the theory and mechanism of adhesive resin bonding to dentin: a critical review. J Biomed Mater Res B Appl Biomater. 2009;88(2):558-578.
50. Helvey GA. The history of adhesive bonding. Dentalaegis web site. cde.dentalaegis.com/courses/4508-the-history-of-adhesive-bonding. Accessed October 12, 2011.
51. Kanca J III. Bonding to tooth structure: a rational rationale for a clinical protocol. J Esthet Dent. 1989;1(4):135-138.
52. Bertolotti RL. Total etch—the rational dentin bonding protocol. J Esthet Dent. 1991;3(1):1-6.
53. Bassiouny M, Ying L. Adhesive compatibility of restorative resins with dentin bonding agents [abstract]. J Dent Res. 1984;63:232.
54. Broome JC. Duke ES, Norling BK. Shear bond strengths of composite resins with three different adhesives [abstract]. J Dent Res. 1985;64:244.
55. Garg N, Garg A. Textbook of Operative Dentistry. New Delhi, India: Jaypee Brothers Medical Publishers; 2010:246.
56. Pashley DH, Livingston MJ. Effect of molecular size on permeability coefficients in human dentine. Arch Oral Biol. 1978;23(5):391-395.
57. Nakabayashi N, Kojima K, Masuhara E. The promotion of adhesion by the infiltration of monomers into tooth substrates. J Biomed Mater Res. 1982;16(3):265-273.
58. Nakabayashi N. Bonding of restorative materials to dentine: the present status in Japan. Int Dent J. 1985;35(2):145-154.
59. Kanca J III. A method for bonding to tooth structure using phosphoric acid as a dentin-enamel conditioner. Quintessence Int. 1991;22(4):285-290.
60. Gwinnett AJ. Moist versus dry dentin: its effect on shear bond strength. Am J Dent. 1992;5(3):127-129.
61. Sugizaki J. The effects of various primers on dentin adhesion of adhesive composites. Jpn T Conserv Dent. 1991;34:228-265.
62. Pashley DH. The evolution of dentin bonding. Dent Today. 2003;22(5):112-119.
63. Gwinnett AJ. Dentin bond strength after air drying and rewetting. Am J Dent. 1994;7(3):144-148.
64. Perdigão J, Geraldeli S. Bonding characteristics of self-etching adhesives to intact versus prepared enamel. J Esthet Restor Dent. 2003;15(1):32-41.
65. Perdigão J, Geraldeli S, Hodges JS. Total-etch versus self-etch adhesive: effect on postoperative sensitivity. J Am Dent Assoc. 2003;134(12):1621-1629.
66. Tay FR, Gwinnett AJ, Pang KM, Wei SH. Structural evidence of a sealed tissue interface with a total-etch wet-bonding technique in vivo. J Dent Res. 1994;73(3):629-636.
67. Söderholm KJ. Dental adhesives…how it all started and later evolved. J Adhes Dent. 2007;9(Suppl 2):227-230.
68. Alhadainy HA, Abdalla AI. 2-year clinical evaluation of dentin bonding systems. Am J Dent. 1996:9(2):77-79.
69. Brunton PA, Cowen AJ, Wilson MA, Wilson NH. A three-year evaluation of restorations placed with a smear-layer-mediated dentin bonding agent in non-carious cervical lesions. J Adhes Dent. 1999;1(4):333-341.
70. Mandras RS, Thurmond JW, Latta M, et al. Three-year clinical evaluation of the Clearfil Liner Bond system.Oper Dent. 1997;22(6):266-270.
71. Ferrari M, Mannocci F, Vichi A, Davidson CL. Effect of two etching times on the sealing ability of Clearfil Liner Bond 2 in Class V restorations. Am J Dent. 1997;10(2):66-70.
72. Yoshiyama M, Sano H, Carvalho RM, Pashley DH. Adhesive mechanism of a self-etching/self-priming adhesive resin to human enamel and dentin. J Hard Tiss Biol. 1996;5(1):31-35.
73. Pashley EL, Comer RW, Simpson MD, et al. Dentin permeability: sealing the dentin in crown preparations.Oper Dent. 1992;17(1):13-20.
74. Helvey GA. Prehybridization: an alternative method for dentin bonding. Dent Today. 2000;19(12):82-87.
75. Paul SJ, Schärer P. The dual bonding technique: a modified method to improve adhesive luting procedures. nt J Periodontics Restorative Dent. 1997;17(6):536-545.
76. Okuda M, Nikaido T, Maruoka R, et al. Microtensile bond strengths to cavity floor dentin in indirect composite restorations using resin coating. J Esthet Restor Dent. 2007;19(1):38-46.
77. Magne P, Kim TH, Cascione D, Donovan TE. Immediate dentin sealing improves bond strength of indirect restorations. J Prosthet Dent. 2005;94(6):511-519.
78. Bertschinger C, Paul SJ, Lüthy H, Schärer P. Dual application of dentin bonding agents: effect on bond strength. Am J Dent. 1996;9(3):115-119.
79. Dietschi D, Monasevic M, Krejci I, Davidson C. Marginal and internal adaptation of class II restorations after immediate or delayed composite placement. J Dent. 2002;30(5-6):259-269.
80. Stavridakis MM, Krejci I, Mange P. Immediate dentin sealing of onlay preparations: thickness of pre-cured Dentin Bonding Agent and effect of surface cleaning.Oper Dent. 2005;30(6):747-757.
81. Dillenburg AL, Soares CG, Paranhos MPG, et al. Microtensile bond strength of prehybridized dentin: storage time and surface treatment effects. J Adhes Dent. 2009;11(3):231-237.
82. de Andrade OS, de Goes MF, Montes MA. Marginal adaptation and microtensile bond strength of composite indirect restorations bonded to dentin treated with adhesive and low-viscosity composite. Dent Mater. 2007;23(3):279-287.
83. Jayasooriya PR, Pereira PN, Nikaido T, et al. The effect of a "resin-coating" on the interfacial adaptation of composite inlays.Oper Dent. 2003;28(1):28-35.
84. Nikaido T, Cho E, Nakajima M, et al. Tensile bond strengths of resin cements to bovine dentin using resin coating. Am J Dent. 2003;16(Spec No.):41A-46A.
85. Dietschi D, Olsburg S, Krejci I, Davidson C. In vitro evaluation of marginal and internal adaptation after occlusal stressing of indirect class II composite restorations with different resinous bases. Eur J Oral Sci. 2003;111(1):73-80.
86. Van Meerbeek B, Vargas M, Inoue S, et al. Adhesives and cements to promote preservation dentistry.Oper Dent. 2001;26(Suppl 6):S119-S144.
87. Magne P, Magne M, Belser UC. Adhesive restorations, centric relation, and the Dahl principle: minimally invasive approaches to localized anterior tooth erosion. Eur J Esthet Dent. 2007;2(3):260-273.
88. Powers JM, Farrah JW. Technique sensitivity in bonding enamel and dentin. Compend Contin Educ Dent. 2010;31(Spec No. 3):1-9.
89. Opdam NJ, Feilzer AJ, Roeters JJ, Smale I. Class I occlusal composite resin restorations: in vivo post-operative sensitivity, wall adaptation, and microleakage. Am J Dent. 1998;11(5):229-234.
90. Craig RG, Powers JM, Wataha JC, eds. Dental Materials: Properties and Manipulation. 9th ed. St Louis, MO: Mosby Elsevier; 2008.
91. Söderholm KJ, Guelmann M, Bimstein E. Shear bond strength of one 4th and two 7th generation bonding agents when used by operators with different bonding experience. J Adhes Dent. 2005;7(1):57-64.
92. Alex G, Leinfelder KF, Swift E Jr. Are three-step total-etch systems still the gold standard? Inside Dentistry2008;4(10):72-73.
93. Wilder AD Jr, Swift EJ Jr, Heymann HO, et al. A 12-year clinical evaluation of a three-step dentin adhesive in noncarious cervical lesions. J Am Dent Assoc. 2009;140(5):526-535.
94. Grewal MS, Grewal SB, Sharm N. Bonding systems: present and future. JIDA. 2011;5(5):656-658.
95. Rotta M, Bresciani P, Moura SK, et al. Effects of phosphoric acid pretreatment and substitution of bonding resin on bonding effectiveness of self-etching systems to enamel. J Adhes Dent. 2007;9(6):537-545.
96. Tay FR, Pashley DH, Peters MC. Adhesive permeability affects composite coupling to dentin treated with a self-etch adhesive.Oper Dent. 2003;28(5):610-621.
97. Kenshima S, Reis A, Uceda-Gomez N, et al. Effect of smear layer thickness and pH of self-etching adhesive systems on the bond strength and gap formation to dentin. J Adhes Dent. 2005;7(2):117-126.
98. Pashley DH, Tay FR. Aggressiveness of contemporary self-etching adhesives. Part II: etching effects on unground enamel. Dent Mater. 2001;17(5):430-444.
99. Lührs AK, Guhr S, Schilke R, et al. Shear bond strength of self-etch adhesives to enamel with additional phosphoric acid etching.Oper Dent. 2008;33(2):155-162.
100. Kallenos TN, Al-Badawi E, White GE. An in vitro evaluation of microleakage in class I preparations using 5th, 6th and 7th generation composite bonding agents. J Clin Pediatr Dent. 2005;29(4):323-328.
101. Watanabe T, Tsubota K, Takamizawa T, et al. Effect of prior acid etching on bonding durability of single-step adhesives.Oper Dent. 2008;33(4):426-433.
102. Brackett WW, Ito S, Nishitani Y, et al. The microtensile bond strength of self-etching adhesives to ground enamel.Oper Dent. 2006;31(3):332-337.
103. Ibrahim IM, Elkassas DY, Yousry MM. Effect of EDTA and phosphoric acid pretreatment on the bonding effectiveness of self-etch adhesives to ground enamel. Eur J Dent. 2010;4(4):418-428.
104. Christensen GJ. Making Class II resin-based composite restorations predictable and profitable. J Am Dent Assoc. 2010;141(4):457-460.
105. Ritter R. Using selective etch technique for esthetic restorations. Inside Dentistry. 2011;7(3):102-103.
106. Rotta M, Bresciani P, Moura SK, et al. Effects of phosphoric acid pretreatment and substitution of bonding resin on bonding effectiveness of self-etching systems to enamel. J Adhes Dent. 2007;9(6):537-545.
107. Van Landuyt K, Kanumilli P, De Munck J, et al. Bond strength of a mild self-etch adhesive with and without prior acid-etching. J Dent. 2006;34(1):77-85.
108. Ikeda M, Tsubota K, Takamizawa T, et al. Bonding durability of single-step adhesives to previously acid-etched dentin.Oper Dent. 2008;33(6):702-709.
109. Torii Y, Itou K, Nishitani Y, et al. Effect of phosphoric acid etching prior to self-etching primer application on adhesion of resin composite to enamel and dentin. Am J Dent. 2002;15(5):305-308.
110. Perdigão J, Gomes G, Lopes MM. Influence of conditioning time on enamel adhesion. Quintessence Int. 2006;37(1):35-41.
111. Magne P, Nielsen B. Interactions between impression materials and immediate dentin sealing. J Prosthet Dent. 2009;102(5):298-305.
About the Author
Gregg A. Helvey, DDS
Adjunct Associate Professor
Virginia Commonwealth University School of Dentistry
Richmond, Virginia
Private Practice
Middleburg, Virginia