You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
For many years there has been controversy as to whether keratinized gingiva (KG) is necessary to maintain health around teeth,1,2 and this debate persists with respect to the role of keratinized tissue adjacent to dental implants.3-5 Historically, an increased zone of keratinized tissue was presumed desirable for the following reasons: provides a resistant barrier to plaque-induced inflammation6; replaces unkeratinized margins to prevent recession7; deepens vestibules to provide better access for toothbrushing8; dissipates functional and masticatory stress placed on the gingival margin of a restoration9; and improves esthetics, patient comfort, and ease of hygiene.4,10,11
In response to perceived needs for KG, surgical techniques were devised to augment gingiva around teeth and implants.12 These procedures continue to be performed because of the persistent disagreement concerning the requirement for keratinized tissue to maintain health. The objective of this paper is to appraise the data and provide a perspective as to why generalized conclusions can or cannot be made regarding the need for KG to sustain health around dental implants. First, data related to the relationship between keratinized tissue and teeth are reviewed. Then anatomic differences in tissues surrounding teeth and implants are addressed. These data serve as background information for the discussion concerning the relevance of KG in maintaining peri-implant health. To avoid confusion about anatomic and histological terminology, definitions of terms used in this review are provided in Table 1. The specific type of tissue referred to in studies will be stated. Note that the terms keratinized mucosa and keratinized gingiva represent the same type of tissue and may include attached and unattached gingiva.
Keratinized Tissue around Teeth
Numerous authors suggested that a lack of keratinized tissue predisposed the periodontium around teeth to deterioration, because they believed KG was more resistant to periodontal destruction than alveolar mucosa.6-8 To assess the validity of this assumption, clinical trials were performed to evaluate whether a band of KG was necessary to maintain health. In 1972, Lang and Löe13 published the first controlled clinical trial that examined the relationship between the width of KG and gingival health. They reported that 80% of tooth surfaces with > 2 mm of KG were healthy. However, all surfaces with < 2 mm of KG manifested signs of clinical inflammation. It was concluded that 2 mm of KG was needed to maintain gingival health (1 mm attached gingiva and 1 mm unattached). Subsequently, numerous investigations were conducted to verify if keratinized tissue was needed to maintain periodontal health.
Clinical trials indicated that sites with a minimal amount of KG9 or attached gingiva14-17 could remain healthy and that increasing the zone of keratinized tissue did not influence periodontal status.15-17 Furthermore, investigators demonstrated17,18 that reducing inflammation was sufficient to maintain clinical attachment levels regardless of the keratinized tissue’s width. Histologic confirmation that the size of the attached gingiva was noncontributory to maintaining health or avoiding recession was provided in a dog model,19 a finding that was confirmed in a study among humans.20
Long-term data with respect to the stability of margins with a lack of KG has been documented. In a group of students with a high degree of oral hygiene, areas with inadequate zones of attached gingiva were maintained (4 to 18 years) without further recession.21-23 In contrast, Agudio et al24 reported 10- to 27-year data (means 15.3 years) with respect to two groups of patients and noted that sites with a lack of attached gingiva manifested a mean recession of 0.7 mm and 1 mm, respectively.
Concerning restorations with subgingival margins, Stetler and Bissada25 found that teeth with narrow zones (< 2 mm) of keratinized mucosa demonstrated a significantly higher gingival index (GI) than teeth with wide zones of KG (> 2 mm). Similarly, in beagle dogs, Ericsson and Lindhe26 reported that subgingival crown margins placed in areas with no KG that were allowed to accumulate plaque over months were prone to develop larger cellular infiltrates than sites with KG. These data conflict with the histological report by Wennström et al19 that indicated there was no difference in the inflammatory response with respect to the amount of attached gingiva present.
In summary, the preponderance of evidence indicates that augmenting KG around teeth should be approached cautiously. The patient’s dental history and chronicity of problems should be assessed before performing gingival augmentation. Measurements should periodically be taken from the cemento-enamel junction (CEJ) to the free gingival margin (FGM) to determine if recession occurred, or from the CEJ to the base of the pocket to determine if loss of clinical attachment occurred.27 Ultimately, the status of tissues should be the critical determinant as to whether KG should be augmented; this decision should not be based upon an assumption that augmenting KG is essential to maintaining periodontal health. These conclusions are in accord with the European Workshop on Periodontology.1 The participants reached the consensus that treatment for the sole purpose of increasing the apicocoronal width of the gingiva to maintain periodontal health and prevent the development of soft-tissue recession around teeth cannot be justified.1 Subsequent to this report there have been no data that challenged the conclusions of the workshop with respect to teeth.
Keratinized Tissue around Implants
Differences in Periodontal Tissues Surrounding Teeth and Implants
Teeth and dental implants have either gingiva or mucosa adjacent to them as they emerge into the oral cavity. Within the crevice around these structures the oral sulcular epithelium is connected to the junctional epithelium (JE). The JE on average is 1-mm long28 and is attached to teeth and dental implants by hemi-desmosones.29 Subjacent to the JE around teeth is a connective tissue layer about 1-mm long. Around teeth, this layer contains fibers that are perpendicular to the root surfaces and insert into the cementum. Surrounding implants, the connective tissue fibers are parallel or oblique30 and do not insert into the implant surface. In addition, the blood supply around implants is less than around teeth, because the periodontal ligament is absent.31
Animal Models: Histological Response to Plaque Accumulation
Due to anatomical differences, peri-implant and periodontal tissues may differ in their response to bacterial infections.26,32 Ericsson et al26 reported that after cessation of hygiene in a dog model the apical extent of the inflammatory infiltrate was larger in peri-implant mucosa. Similarly, in a dog model when ligatures were placed to induce destruction, there was greater bone loss around implants than teeth.32 It was suggested that peri-implant mucosa might be more susceptible to destruction than tissues around teeth. In this regard, when Warrer et al33 placed ligatures around implants (in monkeys) at sites with and without adjacent keratinized mucosa, they noted that locations without keratinized tissue demonstrated more recession and attachment loss than sites with keratinized tissue. In contrast, Strub et al34, in a dog model, failed to find differences in peri-implant soft-tissue recession or bone loss between sites with and without attached gingiva.
Clinical Trial Results in Humans: Importance of KG
Concerning the effect of a dearth of KG, there is conflicting information in the literature regarding six issues: implant survivability, plaque accumulation, tissue inflammation, recession, probing depths, and bone loss. These issues are discussed separately, because within a given study a lack of KG may negatively affect only one parameter listed above and not others. Furthermore, it is recognized that numerous variables that have not been accounted for in many investigations (eg, biotype, smoking, surgical management of implant area, occlusal forces, etc.) can affect study results.
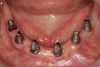
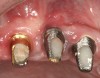
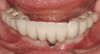
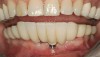
There are several other issues that should be noted with respect to studies that addressed the role of KG around implants. Researchers did not differentiate between attached and unattached gingiva. This is probably due to the fact that probing depths are usually deeper around implants than healthy teeth because of differences in the anatomy. In addition, there is difficulty in detecting the coronal level of the biologic width, because the probe penetrates into the JE and this results in underestimating the amount of attached gingiva present.35 Furthermore, measurements are usually taken on the buccal aspect, and studies have not related clinical parameters to interproximal tissues. It should also be noted that when all of the gingiva is excised in a dog model, the marginal tissue that reforms usually has a keratinized margin that is not attached.19 Therefore, when there is a lack of KG, the typical finding is a keratinized margin with mucosa (Figure 1), and only rarely is a pure mucosal margin found (Figure 2).
Long-Term Implant Survivability
Based on long-term implant studies, numerous investigators concluded that there was little or no difference in the survival rate for implants surrounded by oral mucosa or KG.36-40 These data pertain to smooth-surfaced implants and screw-retained prostheses. On the other hand, two investigations demonstrated there was a lower implant survival rate when keratinized tissue was absent around textured-surfaced implants. This occurred around hydroxyapatite (HA)-coated implants41 and IMZ plasma-sprayed implants.42 However, confounding variables include the fact that HA-coated implants may manifest a lower long-term survival rate than noncoated implants43-45 and plasma-sprayed implants have been replaced with sandblasted/acid-etched (SLA)-surfaced implants.46,47
Currently, textured-surfaced implants are employed. In this regard, Abrahamsson et al48 reported that the histological attachment of the tissue (JE and connective tissue) adjacent to an implant was consistent irrespective of the type of implant or surface roughness.49 However, they also indicated that plaque control was easier on smooth-surfaced implants compared to textured surfaces.48 Therefore, the following question arises: If it is harder to cleanse textured surfaces than smooth surfaces and most of the above survival data pertains to smooth surfaces, is it appropriate to extrapolate the above findings to textured surfaces? To definitively answer that question, additional long-term studies are needed to assess whether textured-surfaced implants will manifest high survival rates over a long period of time despite a dearth of KG.
Plaque Index
Three studies reported a higher plaque index (PI) around implants associated with < 2 mm of KG compared to > 2 mm KG (Table 2).4,10,50 Ostensibly, this was due to the tissues being movable or they were more tender to brush, so sites were avoided during oral hygiene. Others did not corroborate these findings.28,50-52 Schrott et al50 reported higher plaque readings on the lingual but not the buccal when both sides manifested a dearth of keratinized tissue.
Inflammation
It was suggested that KG tissue is more protective than mucosa and inhibits an inflammatory alteration in the connective tissue around implants.48 In this regard, when there was a dearth of keratinized tissue (< 2 mm), several investigators reported there was a statistically significant increase in the amount of inflammation (GI or percentage of sites manifesting bleeding upon probing) compared to sites with > 2 mm KG (Figure 3) (Table 2).4,11,53 Others reported the amount of inflammation was not increased when there was a lack of KG (Figure 4).28,51,52
Recession
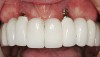
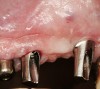
Some researchers found that the absence of KG was associated with a statistically significant increased amount of recession around implants in humans (Figure 5) (Table 2),50,51,53 but this finding conflicted with data of others (Figure 6 and Figure 7).28,54 Brägger et al52 found a weak correlation between the amount of KG and recession. In addition, it should be noted that in the study by Schrott et al50 the standard deviation around the means was greater than the mean data, which raises questions as to the accuracy of these findings regarding recession (for < 2 mm vs. > 2 mm KG, recession was, respectively, 0.69 +/- 1.11 mm vs. 0.08 +/- 0.86 mm). Furthermore, it needs to be noted that a lack of attached gingiva may not be the precipitating factor for ongoing deterioration of a site, but rather a consequence of recession.
Probing Depths
Most studies concluded that a lack of KG was not associated with increased probing depths (Table 2)4,11,51; however, one study reported contradictory data.53
Bone or Clinical Attachment Loss
Several clinical trials concluded that the absence of KG was associated with a statistically significant increase in bone loss (Table 2)4,41,51 or attachment loss53 when sites with and without KG were compared, but others did not corroborate this finding.11,55 The study by Chung et al11 also noted that there was no association between bone loss and absence of KG and these data pertain to different implant surface configurations (eg, smooth, textured). Several other factors need to be considered when interpreting these data. It should be noted that the mean differences between control and test groups with respect to bone loss reported by Kim et al51 and Bouri et al4 were only 0.34 mm51 and 0.28 mm,4 respectively. Furthermore, in all of the previous studies, assessments of bone loss were done on nonstandardized radiographs and the data by Block et al41 were determined on HA-coated dental implants, which were prone to bone loss and failure.
The Role of KG around Implants Supporting Overdentures
Adibrad et al56 reported that when implants supporting an overdenture with < 2 mm KG surrounding them were compared to implants with > 2 mm KG, sites with < 2 mm of keratinized tissue were associated with higher plaque accumulation, gingival inflammation, bleeding upon probing, and mucosal recession. It was speculated that the flange of overdentures may favor plaque accumulation and mucosal irritation.56 In contrast, Heckman et al57 noted no significant difference was found between bleeding scores at sites with and without KG under an overdenture. Similarly, Kaptein et al58 reported no difference in probing depth, plaque, or bleeding around implants with respect to the width of KG under overdentures. Others also found there was no detrimental effect around implants that supported overdentures if there was a lack of KG.40,59-61
Discussion
It was anticipated that a comprehensive inspection of the literature would provide an unambiguous answer to the question of whether or not KG around dental implants is necessary to maintain peri-implant health. However, the literature provided contradictions with respect to every facet of this review, which precludes drawing definitive conclusions. In addition, the literature pertaining to dental implants failed to provide any clarity as to how much KG is necessary to maintain health. It appears there may be circumstances where it is beneficial to have keratinized tissue and others where it is unnecessary, and this may vary in different patients and sites within the same mouth. However, defining specific situations adjacent to implants (eg, clefting of gingiva, recession, shallow vestibule, frenum pull) or for particular patients (eg, thick or thin biotypes) that may benefit from gingival augmentation has never been investigated. Instead, studies consistently employed test and control groups with respect to either a dearth or an adequate band of KG. To provide a perspective as to what information can be derived from studies and clinical experience, the impact of a lack of keratinized tissue on peri-implant health is discussed from several points of view: periodontal response to plaque adjacent to crown margins on teeth and implants, overall statistical significance of study findings, and interpretation and practical application of data to patient management.
In general, subgingival crown margins on teeth predispose patients to increased plaque accumulation and greater amounts of gingival inflammation than teeth without crowns.25,62-65 Hypothetically, the inflammatory reaction around implant restorations may be worse than around teeth, because tissues surrounding dental implants may be more susceptible to inflammation due to the altered structure of connective around an implant.1,26,30 However, this hypothesis is based upon animal studies that assessed the peri-implant response when hygiene is ceased for months or if ligatures were placed. These artificially created situations can provide some proof of principle concepts, but these data cannot be directly extrapolated to patient management. Furthermore, it needs to be noted that individual thresholds of plaque necessary to trigger signs of disease may vary between patients.66 Most importantly, investigators reported that the response of tissues to plaque in patients was similar around teeth and implants,67,68 and tissue response around implants was not problematic even when there was dearth of KG.20,51,52 Based on these studies and the above clinical trials relating the level of plaque to the amount of KG, it cannot be concluded that all patients are more prone to plaque accumulation due to a lack of KG.
Assessment of the clinical trials relating clinical parameters to the amount of KG can provide a perspective concerning trends that have been observed. Inspection of data in Table 2 suggests that in some studies there is a tendency to find increased inflammation, recession, and bone loss associated with diminished amounts of keratinized tissue. However, it is important to note that statistically significant findings only indicate that the event did not occur by chance. It does not mean that the outcome was large or important.69 Therefore, clinicians need to decide whether findings in Table 2 are clinically significant. Unfortunately, the definition of clinical significance varies depending on the following factors: specific clinical field being addressed, size of the effect, measurement used to evaluate a therapy, and, ultimately, the clinical importance of the findings.70 With respect to the relationship between clinical parameters and absence/presence of KG, a possible explanation for inconsistency in the literature is that with good oral hygiene, peri-implant soft-tissue health can usually be maintained if keratinized tissue surrounding implant/restoration is absent.5 However, if there is less than good oral hygiene, it may be advantageous to have KG.5 Use of the “good hygiene” criterion is complicated by the fact that defining a threshold that would be considered less than “good hygiene” is problematic.
After reviewing the literature, Yeung5 suggested that a case could be made for augmenting keratinized tissue around implants to facilitate plaque control by reducing mobile mucosal tissue. To bolster this contention, he referred to a consensus of opinions expressed by authors who participated in a debate as to whether KG was needed around implants. The authors supported the practice of augmenting keratinized tissue around implant restorations when practical to minimize complications.71 In contrast, Esposito et al72 concluded in a systematic review that there was insufficient evidence to recommend increasing KG to maintain peri-implant health. Since publication of that systematic review several clinical trials were published.4,11,50,51,53 They provided mixed results with respect to the necessity of augmenting keratinized tissue when comparing studies, and within the same investigation the absence of KG may or may not have an effect on certain clinical parameters. Perplexingly, the data can be interpreted opposite ways. It could be concluded that a strong case cannot be made for routine augmentation of keratinized tissue around dental implants when there is dearth of KG, because there is inconsistency between studies to indicate that it is detrimental to restore implants when there is a lack of KG. Furthermore, when there were differences in the response of clinical parameters at sites with or without KG, the mean differences were usually small. In contrast, it could be contended that recent investigations indicated additional bone4,51 or attachment loss53 was associated with a dearth of keratinized tissue. While the increased mean bone loss was usually small (Table 2), there may be sites where bone loss was substantial. However, frequency distributions were not provided; therefore, it is not possible to distinguish how often and to what extent this occurred or if the data was skewed by a few sites that had extensive bone loss. It could also be asserted that short-term data (Table 2) may not reflect the possibility that an inflamed site may continue to deteriorate and compromise a restoration.
Apparently, for some patients a lack of KG may be a risk factor for one or more issues: plaque accumulation, tissue soreness while brushing, increased gingival inflammation, recession, and bone loss. In this regard, studies are needed to provide predictive values with respect to how often specific types of biological problems occur when KG is lacking. Ultimately, when there is a lack of keratinized tissue, clinicians need to make a decision to augment or not to increase the band of KG at a site for that particular individual based on data in the literature, the patient’s dental history, the unique characteristics of the site being treated, and the clinician’s experiences.
The literature does not clearly define a patient’s susceptibility to deterioration in the absence of KG; however, there are situations where it seems logical that augmentation of KG would be beneficial:
- Chronically inflamed sites, despite oral hygiene instruction and periodontal therapy. Sometimes it is necessary to alter the gingival topography to make hygiene easier.
- Locations with ongoing recession or continued loss of clinical attachment or bone, regardless of periodontal therapy and good oral hygiene.
- Sites where the patient complains of soreness when brushing, despite the appearance of gingival health.
- Dental history suggesting predisposition to periodontitis or recession.
- Patients noncompliant with periodic professional maintenance.
- To improve esthetics.
References
1. Lindhe J, Echeverria J. Consensus report of session II. In: Lang NP, Karring T, eds. Proceedings of the 1st European Workshop on Periodontology. Berlin, Germany: Quintessence Publishing; 1994:210-214.
2. Marquez IC. The role of keratinized tissue and attached gingiva in maintaining periodontal/peri-implant health. Gen Dent. 2004;52(1):74-78.
3. Cairo F, Pagliaro U, Nieri M. Soft tissue management at implant sites. J Clin Periodontol. 2008;35(8 suppl):163-167.
4. Bouri A Jr, Bissada N, Al-Zahrani MS, et al. Width of keratinized gingiva and the health status of the supporting tissues around dental implants. Int J Oral Maxillofac Implants. 2008;23(2):323-326.
5. Yeung SC. Biological basis for soft tissue management in implant dentistry. Aust Dent J. 2008;53(suppl 1):S39-S42.
6. Maynard JG Jr, Ochsenbein C. Mucogingival problems, prevalence and therapy in children. J Periodontol. 1975;46(9):543-552.
7. Sullivan HC, Atkins JH. Free autogenous gingival grafts. 3. Utilization of grafts in the treatment of gingival recession. Periodontics. 1968;6(4):152-160.
8. Nabers JM. Extension of the vestibular fornix utilizing a gingival graft—case history. Periodontics. 1966;4(2):77-79.
9. Miyasato M, Crigger M, Egelberg J. Gingival condition in areas of minimal and appreciable width of keratinized gingiva. J Clin Periodontol. 1977;4(3):200-209.
10. Chang M, Odman PA, Wennström JL, Andersson B. Esthetic outcome of implant-supported single-tooth replacements assessed by the patient and by prosthodontists. Int J Prosthodont. 1999;12(4):335-341.
11. Chung DM, Oh TJ, Shotwell JL, et al. Significance of keratinized mucosa in maintenance of dental implants with different surfaces. J Periodontol. 2006;77(8):1410-1420.
12. Thoma DS, Benić GI, Zwahlen M, et al. A systematic review assessing soft tissue augmentation techniques. Clin Oral Implants Res. 2009;20(suppl 4):146-165.
13. Lang NP, Löe H. The relationship between the width of keratinized gingiva and gingival health. J Periodontol. 1972;43(10):623-627.
14. Tenenbaum H. A clinical study comparing the width of attached gingiva and the prevalence of gingival recessions. J Clin Periodontol. 1982;9(1):86-92.
15. Hangorsky U, Bissada NF. Clinical assessment of free gingival graft effectiveness on the maintenance of periodontal health. J Periodontol. 1980;51(5):274-278.
16. de Trey E, Bernimoulin JP. Influence of free gingival grafts on the health of the marginal gingiva. J Clin Periodontol. 1980;7(5):381-393.
17. Dorfman HS, Kennedy JE, Bird WC. Longitudinal evaluation of free autogenous gingival grafts. A four year report. J Periodontol. 1982;53(6):349-352.
18. Kennedy JE, Bird WC, Palcanis KG, Dorfman HS. A longitudinal evaluation of varying widths of attached gingiva. J Clin Periodontol. 1985;12(8):667-675.
19. Wennström J, Lindhe J. Role of attached gingiva for maintenance of periodontal health. Healing following excisional and grafting procedures in dogs. J Clin Periodontol. 1983;10(2):206-221.
20. Wennström JL. Lack of association between width of attached gingiva and development of soft tissue recession. A 5-year longitudinal study. J Clin Periodontol. 1987;14(3):181-184.
21. Salkin LM, Freedman AL, Stein MD, Bassiouny MA. A longitudinal study of untreated mucogingival defects. J Periodontol. 1987;58(3):164-166.
22. Freedman AL, Green K, Salkin LM, et al. An 18-year longitudinal study of untreated mucogingival defects. J Periodontol. 1999;70(10):1174-1176.
23. Freedman AL, Salkin LM, Stein MD, Green K. A 10-year longitudinal study of untreated mucogingival defects. J Periodontol. 1992;63(2):71-72.
24. Agudio G, Nieri M, Rotundo R, et al. Periodontal conditions of sites treated with gingival-augmentation surgery compared to untreated contralateral homologous sites: a 10- to 27-year long-term study. J Periodontol. 2009;80(9):1399-1405.
25. Stetler KJ, Bissada NF. Significance of the width of keratinized gingiva on the periodontal status of teeth with submarginal restorations. J Periodontol. 1987;58(10):696-700.
26. Ericsson I, Berglundh T, Marinello C, et al. Long-standing plaque and gingivitis at implants and teeth in the dog. Clin Oral Implants Res. 1992;3(3):99-103.
27. Greenstein G. Advances in periodontal disease diagnosis. Int J Periodontics Restorative Dent. 1990:10(5):350-375.
28. Gargiulo AW, Wentz FM, Orban B. Dimensions and relations of the dentogingival junction in humans. J Periodontol. 1961;32:261-267.
28. Wennström JL, Bengazi F, Lekholm U. The influence of the masticatory mucosa on the peri-implant soft tissue condition. Clin Oral Implants Res. 1994;5(1):1-8.
29. Shimono M, Ishikawa T, Enokiya Y, et al. Biological characteristics of the junctional epithelium. J Electron Microsc (Tokyo). 2003;52(6):627-639.
30. Berglundh T, Lindhe J, Ericsson I, et al. The soft tissue barrier at implants and teeth. Clin Oral Implants Res. 1991;2(2):81-90.
31. Berglundh T, Lindhe J, Jonsson K, Ericsson I. The topography of the vascular systems in the periodontal and peri-implant tissues in the dog. J Clin Periodontol. 1994;21(3):189-193.
32. Lindhe J, Berglundh T, Ericsson I, et al. Experimental breakdown of peri-implant and periodontal tissues. A study in the beagle dog. Clin Oral Implants Res. 1992;3(1):9-16.
33. Warrer K, Buser D, Lang NP, Karring T. Plaque-induced peri-implantitis in the presence or absence of keratinized mucosa. An experimental study in monkeys. Clin Oral Implants Res. 1995;6(3):131-138.
34. Strub JR, Gaberthuel TW, Grunder U. The role of attached gingiva in the health of peri-implant tissue in dogs. 1. Clinical findings. Int J Periodontics Restorative Dent. 1991;11(4):317-333.
35. Polson AM, Caton JG, Yeaple RN, Zander HA. Histological determination of probe tip penetration into the gingival sulcus of humans using an electronic pressure-sensitive probe. J Clin Periodontol. 1980;7(6):479-488.
36. Brånemark PI, Svensson B, van Steenberghe D. Ten-year survival rates of fixed prostheses on four or six implants ad modum Brånemark in full edentulism. Clin Oral Implants Res. 1995;6(4):227-231.
37. Buser D, Mericske-Stern R, Bernard JP, et al. Long-term evaluation of non-submerged ITI implants. Part 1: 8-year life table analysis of a prospective multi-center study with 2359 implants. Clin Oral Implants Res. 1997;8(3):161-172.
38. Jemt T, Chai J, Harnett J, et al. A 5-year prospective multicenter follow-up report on overdentures supported by osseointegrated implants. Int J Oral Maxillofac Implants. 1996;11(3):291-298.
39. Lindquist LW, Carlsson GE, Jemt T. A prospective 15-year follow-up study of mandibular fixed prostheses supported by osseointegrated implants. Clinical results and marginal bone loss. Clin Oral Implants Res. 1996;7(4):329-336.
40. Mericske-Stern R. Clinical evaluation of overdenture restorations supported by osseointegrated titanium implants: a retrospective study. Int J Oral Maxillofac Implants. 1990;5(4):375-383.
41. Block MS, Gardiner D, Kent JN, et al. Hydroxyapatite-coated cylindrical implants in the posterior mandible: 10-year observations. Int J Oral Maxillofac Implants. 1996;11(5):626-633.
42. Kirsch A, Ackermann KL. The IMZ osteointegrated implant system. Dent Clin North Am. 1989;33(4):733-791.
43. Binahmed A, Stoykewych A, Hussain A, et al. Long-term follow-up of hydroxyapatite-coated dental implants—a clinical trial. Int J Oral Maxillofac Implants. 2007;22(6):963-968.
44. Biesbrock AR, Edgerton M. Evaluation of the clinical predictability of hydroxyapatite-coated endosseous dental implants: a review of the literature. Int J Oral Maxillofac Implants. 1995:10(6):712-720.
45. Albrektsson T, Sennerby L. State of the art in oral implants. J Clin Periodontol. 1991;18(6):474-481.
46. De Boever AL, Quirynen M, Coucke W, et al. Clinical and radiographic study of implant treatment outcome in periodontally susceptible and non-susceptible patients: a prospective long-term study. Clin Oral Implants Res. 2009;20(12):1341-1350.
47. Esposito M, Hirsch JM, Lekholm U, Thomsen P. Failure patterns of four osseointegrated oral implant systems. J Mater Sci Mater Med. 1997;8(12):843-847.
48. Abrahamsson I, Berglundh T, Wennström J, Lindhe J. The peri-implant hard and soft tissues at different implant systems. A comparative study in the dog. Clin Oral Implants Res. 1996;7(3):212-219.
49. Abrahamsson I, Zitzmann NU, Berglundh T, et al. The mucosal attachment to titanium implants with different surface characteristics: an experimental study in dogs. J Clin Periodontol. 2002;29(5):448-455.
50. Schrott AR, Jimenez M, Hwang JW, et al. Five-year evaluation of the influence of keratinized mucosa on peri-implant soft-tissue health and stability around implants supporting full-arch mandibular fixed prostheses. Clin Oral Implants Res. 2009;20(10):1170-1177.
51. Kim BS, Kim YK, Yun PY, et al. Evaluation of peri-implant tissue response according to the presence of keratinized mucosa. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107(3):e24-e28.
52. Brägger U, Bürgin WB, Hämmerle CH, Lang NP. Associations between clinical parameters assessed around implants and teeth. Clin Oral Implants Res. 1997;8(5):412-421.
53. Zigdon H, Machtei EE. The dimensions of keratinized mucosa around implants affect clinical and immunological parameters. Clin Oral Implants Res. 2008;19(4):387-392.
54. Bengazi F, Wennström JL, Lekholm U. Recession of the soft tissue margin at oral implants. A 2-year longitudinal prospective study. Clin Oral Implants Res. 1996;7(4):303-310.
55. Roos-Jansaker AM, Renvert H, Lindahl C, Renvert S. Nine- to fourteen-year follow-up of implant treatment. Part III: factors associated with peri-implant lesions. J Clin Periodontol. 2006;33(4):296-301.
56. Adibrad M, Shahabuei M, Sahabi M. Significance of the width of keratinized mucosa on the health status of the supporting tissue around implants supporting overdentures. J Oral Implantol. 2009;35(5):232-237.
57. Heckmann SM, Schrott A, Graef F, et al. Mandibular two-implant telescopic overdentures. Clin Oral Implants Res. 2004;15(5):560-569.
58. Kaptein ML, De Lange GL, Blijdorp PA. Peri-implant tissue health in reconstructed atrophic maxillae—report of 88 patients and 470 implants. J Oral Rehabil 1999;26(6):464-474.
59. Adell R, Lekholm U, Rockler B, Brånemark PI. A 15-year study of osseointegrated implants in the treatment of the edentulous jaw. Int J Oral Surg. 1981;10(6):387-416.
60. Lekholm U, Adell R, Lindhe J, et al. Marginal tissue reactions at osseointegrated titanium fixtures. (II) A cross-sectional retrospective study. Int J Oral Maxillofac Surg. 1986;15(1):53-61.
61. Apse P, Zarb GA, Schmitt A, Lewis DW. The longitudinal effectiveness of osseointegrated dental implants. The Toronto Study: peri-implant mucosal response. Int J Periodontics Restorative Dent. 1991;11(2):94-111.
62. Lang NP, Kiel RA, Anderhalden K. Clinical and microbiological effects of subgingival restorations with overhanging or clinically perfect margins. J Clin Periodontol. 1983;10(6):563-578.
63. Silness JA. Periodontal conditions in patients treated with dental bridges. 3. The relationship between the location of the crown margin and the periodontal condition. J Periodontal Res. 1970;5(3):225-229.
64. Silness J. Periodontal conditions in patients treated with dental bridges. 2. The influence of full and partial crowns on plaque accumulation, development of gingivitis and pocket formation. J Periodontal Res. 1970;5(3):219-224.
65. Marcum JS. The effect of crown marginal depth upon gingival tissue. J Prosthet Dent. 1967;17(5):479-487.
66. Cobb CM. Clinical significance of non-surgical periodontal therapy: an evidence-based perspective of scaling and root planing. J Clin Periodontol. 2002;29(suppl 2):6-16.
67. Zitzmann NU, Berglundh T, Marinello CP, Lindhe J. Experimental peri-implant mucositis in man. J Clin Periodontol. 2001;28(6):517-523.
68. Pontoriero R, Tonelli MP, Carnevale G, et al. Experimentally induced peri-implant mucositis. A clinical study in humans. Clin Oral Implants Res. 1994;5(4):254-259.
69. Greenstein G. Clinical versus statistical significance as they relate to the efficacy of periodontal therapy. J Am Dent Assoc. 2003;134(5):583-591.
70. Lindgren BR, Wielinski CL, Finkelstein SM, Warwick WJ. Contrasting clinical and statistical significance within the research setting. Pediatr Pulmonol. 1993;16(6):336-340.
71. Krygier G, Glick PL, Versman KJ, et al. To minimize complications, is it essential that implant abutments be surrounded by keratinized tissue? Int J Oral Maxillofac Implants. 1997;12(1):127-131.
72. Esposito M, Grusovin MG, Maghaireh H, et al. Interventions for replacing missing teeth: management of soft tissues for dental implants. Cochrane Database Syst Rev. 2007;18;(3):CD006697.
About the Authors
Gary Greenstein, DDS, MS
Clinical Professor
Department of Periodontology
School of Dental Medicine
Columbia University
New York, New York
Private Practice
Surgical Implantology and Periodontics
Freehold, New Jersey
John Cavallaro, DDS
Clinical Director of Dental Implant Fellowship
Clinical Associate Professor
Department of Prosthodontics
School of Dental Medicine
Columbia University
New York, New York
Private Practice
Surgical Implantology and Prosthodontics
Brooklyn, New York