You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
Tissue engineering is broadly defined as the application of engineering and life-science principles to develop biological substitutes that improve or reconstitute organs, tissues, and tissue function.1 Early efforts to engineer periodontal and alveolar bone regeneration relied largely on matrices or scaffolds, including mammalian bone grafts and synthetic bone substitutes, or cell-exclusive materials that compartmentalize the regenerative site, as in guided tissue regeneration (GTR). Bone replacement grafts and barrier technologies have been used in combination; however, membrane placement is often associated with flap dehiscence due to compromised vascularity, which can adversely impact the regenerative outcome.2-5 With the exception of selected autogenous bone grafts and demineralized bone matrix, most bone replacement grafts are generally considered passive scaffolds providing a framework for cellular migration and tissue formation.6 Therefore, bone replacement grafts and GTR membranes appear to function primarily through the preservation of space critical for clot development and tissue maturation. Although current bone grafting and barrier materials support significant clinical improvements, multiple factors appear to influence the robustness and predictability of the regenerative outcome.7,8 Recent attention has focused on the potential for biological mediators to improve wound healing and enhance the clinical benefits of bone replacement grafts.9-12 Preclinical and clinical studies have demonstrated purified and recombinant growth factors, including platelet-derived growth factor (PDGF) and insulin-like growth factor-I (IGF-1), have significant therapeutic potential.13-18 Other recent clinical research has provided compelling evidence that biological mediators offer great potential to enhance the degree and predictability of regenerative outcomes using graft matrices.19-24 In 2005, the Food and Drug Administration (FDA) approved a dental bone-filling device, GEM 21S® (Osteohealth, www.osteohealth.com), which combines recombinant human platelet-derived growth factor-BB (rhPDGF-BB) and ß-tricalcium phosphate (ß-TCP) for the treatment of periodontally related defects. Currently, another recombinant human biologic available for dental surgical procedures is recombinant human BMP-2 (rhBMP-2), which is indicated for sinus elevation procedures and ridge augmentation combined with dental extractions. GEM 21S® is a completely synthetic grafting system for bone and periodontal regeneration composed of highly purified rhPDGF (0.5 mL 0.3 mg/mL) and synthetic ß-TCP (0.5 cc Ca3 [PO4]). Labeled indications include intrabony periodontal defects, furcation periodontal defects, and gingival recession associated with periodontal defects. FDA approval was based on safety and efficacy data from preclinical and clinical studies, including a pivotal large-scale, prospective, randomized, controlled human clinical trial.23
Biologic Effects of PDGF
PDGF exerts potent stimulatory effects as a chemoattractant and mitogen for mesenchymal cells (including osteogenic cells), and promotes angiogenesis, complementing the actions of vascular endothelial growth factor (VEGF) in vessel formation.25,26 PDGF-BB has been shown to enhance the chemotactic and mitogenic activity of periodontal ligament cells at concentrations as low as 1 ng/mL.27,28 PDGF-BB delivered in a methylcellulose gel was reported to have a half-life of 4.2 hours, with greater than 96% clearance of the radiolabeled growth factor by 96 hours, when applied for the treatment of naturally occurring periodontal disease in beagle dogs.17 Following clinical application, the potent actions of this growth factor, therefore, must occur early, triggering a cascade of biologic and cellular events at the surgical wound for weeks. These events are characterized by the recruitment and differentiation of mesenchymal cell populations, as well as new vessel formation, ultimately supporting wound healing and regeneration.25 Cooke et al29 examined the effects of PGDF-BB on levels of VEGF and bone turnover in periodontal wound fluid in 16 patients who were randomized to receive treatment of intrabony defects with either ß-TCP carrier alone, ß-TCP plus 0.3 mg/mL rhPDGF-BB, or ß-TCP plus 1.0 mg/mL rhPDGF-BB. These patients had participated in a large clinical trial evaluating the efficacy and safety of PDGF-BB in the treatment of intraosseous periodontal defects.23 Pyridinoline cross-linked carboxyterminal telopeptide of Type I collagen (ICTP) is an indicator of osseous metabolic activity and provided a marker of bone turnover. Low-dose rhPDGF-BB application was found to elicit increases in ICTP at 3 to 5 days in the wound healing process, with the 1 mg/mL rhPDGF-BB group showing the most pronounced difference in VEGF at 3 weeks. Thus, a single dose of rhPDGF-BB exhibited demonstrable, sustained metabolic actions at the clinical site of application.
In a parallel study, the release of the ICTP into the periodontal wound fluid was monitored longitudinally in 47 patients for 24 weeks following regenerative surgical treatment with PDGF-BB.30 The 0.3 and 1 mg/mL PDGF-BB treatment groups exhibited increases in levels of ICTP for as much as 6 weeks. ICTP levels were significantly higher in defects treated with PDGF-BB and ß-TCP compared with sites grafted with ß-TCP alone at the 6-weeks point. Given the rapid biologic clearance of the growth factor, these results provide further evidence that a single administration of PDGF-BB exerts a sustained effect on periodontal bone metabolism and helps clarify the sequence and timing of signal cascades involved in periodontal wound healing.
Bone Replacement Grafts and rhPDGF in Combination
Currently, PDGF-BB is FDA-approved for periodontal regeneration as part of a dental bone-filling device only. Although this device uses ß-TCP as the scaffold/carrier, there has been considerable clinical interest in combining this growth factor with other bone replacement grafts, particularly bone allografts. Bone allografts, such as freeze-dried bone allograft (FDBA) and demineralized freeze-dried bone allograft (DFDBA), exhibit highly osteoconductive surfaces and support well-documented clinical improvements in periodontal parameters compared to open flap debridement.8 DFDBA has also been shown to possess variable amounts of growth factors, including bone morphogenetic proteins, and the capacity for osteoinduction.31 Because of the safety and efficacy profile of bone allografts, the potential for FDBA and DFDBA to serve as carriers for growth factors and other biologic mediators has been extensively explored and documented in cell-culture and preclinical models.31
Clinical case reports also provide information on the clinical efficacy of PDGF-BB being used with bone allografts. Nevins et al19 and Camelo et al,20 for example, reported human histologic evidence of periodontal regeneration in intra-osseous defects treated employing a combination of rhPDGF-BB and DFDBA. Nevins et al32 reported a case series describing the clinical and radiographic outcomes following the treatment with rhPDGF-BB and FDBA of severe periodontal intrabony defects. Clinical reentry and radiographs at 11 months showed complete bone fill, indicating that rhPDGF combined with FDBA provides excellent clinical results. Preclinical studies and case reports also provide proof of principle that rhPDGF-BB, when combined with other graft matrices, can support improved bone formation and wound healing in alveolar ridge reconstruction and implant therapy. Lynch et al33 found that the direct application of a combination of rhPDGF-BB and IGF-1 around dental implants produced two to three times more new bone at earlier periods in dogs. Becker et al34 reported a twofold increase in the percentage of implant surface in contact with bone and total length of the implant surface in contact with bone in dehiscence defects treated with expanded-polytetrafluoroethylene membranes (ePTFE) plus PDGF/IGF-I compared with the defects receiving ePTFE membranes alone in dogs.34 Simion et al35 reported a canine study that demonstrated the potential for a deproteinized cancellous bovine block, when infused with rhPDGF-BB, to regenerate significant amounts of new bone in severe mandibular vertical ridge defects without placement of a barrier membrane. The xenogenic block grafts were infused with rhPDGF-BB and stabilized in alveolar defects using two dental implants with or without collagen membranes. The alveolar ridge defects treated with the combination of rhPDGF-BB plus xenograft without a collagen membrane demonstrated the greatest bone formation based on radiographic and histologic outcome measures. The histologic findings revealed robust osteogenesis throughout the block grafts, with significant graft resorption and replacement. In contrast, alveolar ridge defects treated with traditional GBR without the growth factor supported little or no bone formation. Simion et al36 reported similar findings using rhPDGF-BB in combination with a novel equine hydroxyapatite and collagen (eHAC) bone block in the canine model. Moreover, recent case reports demonstrate that FDBA and anorganic bovine bone can serve as effective scaffolds to deliver rhPDGF-BB for lateral ridge augmentation and reconstruction, following extraction for implant placement.37,38
Case Reports
Collectively, these preclinical and clinical reports document the potential for rhPDGF-BB, when combined with allogenic and xenogenic grafts, to enhance bone formation and wound healing in alveolar ridge reconstruction. This article reports on clinical experiences using rhPDGF-BB with bone allografts for implant site development. After careful evaluation of clinical parameters and consideration of current and emerging evidence, the off-label use of rhPDGF-BB was determined in the following case to be consistent with good clinical practice and in the patient's best interest. Clinical, radiographic, and histologic observations from the following selected cases are presented to illustrate treatment outcomes achieved using this combinatorial strategy:
- ridge preservation for extraction sockets with alveolar
- wall defects
- ridge preservation for extraction sockets—minimally invasive techniques
- lateral ridge augmentation
- sinus augmentation
All of the cases presented and reviewed were surgically managed using 0.5 mL of 0.3 mg/mL of rhPDGF delivered using a particulate bone allograft (FDBA or DFDBA) as a scaffold. In selected cases, an absorbable membrane was employed to help contain the particulate graft material. The clinical utility of membranes may have a significant benefit when particulate biomaterial scaffolds are chosen as opposed to solid block biomaterials.
Case 1
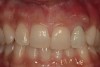
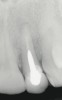
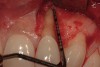
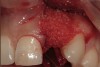
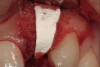
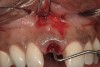
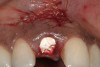
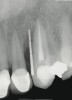
A 34-year-old woman presented with a chief complaint of mild discomfort and swelling associated with the maxillary left lateral incisor. The patient reported a history of root canal therapy and crown performed approximately 10 years earlier. Findings from the clinical examination revealed a localized buccal gingival swelling at the level of the mucogingival junction distal to the lateral incisor, with a 9-mm probing depth on the direct facial of the tooth (Figure 1 ). Observations from the radiographic examination demonstrated a periradicular radiolucency but suggested normal interdental bone levels (Figure 2 ). Surgical exposure following flap elevation exposed a 10-mm dehiscence defect of the buccal plate (Figure 3 ), which was consistent with a vertical root fracture and hopeless prognosis. The lateral incisor was extracted to minimize the loss of alveolar bone and this was followed by thorough degranulation of the socket. Particulate FDBA saturated with rhPDGF-BB for 10 minutes was then applied to augment the deficient ridge in preparation for future implant placement (Figure 4 ). The extraction site was developed to approximate the contours of the adjacent alveolar ridge. Then, a resorbable collagen membrane (Bio-Gide®, Osteohealth) was placed over the graft to facilitate graft containment and maintenance of desired alveolar contours (Figure 5 ). Placement of the membrane without disruption or displacement of the graft during completion of the surgical procedure and suturing is critical to ensure clot formation that will support bone formation.
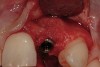
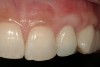
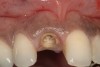
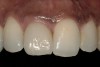
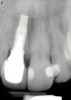
From a clinical perspective, the primary challenge was the development of an esthetic emergence of the dental implant-supported crown restoration. Maintenance of the soft-tissue dimensions, therefore, was critical to the treatment success. In an effort to optimally maintain the ridge form, a connective tissue graft harvested from the palate39,40 was placed over the collagen membrane to augment the soft tissues. Then, the buccal flap was advanced to achieve primary closure. Postoperative healing proceeded uneventfully. The overlying soft tissue exhibited rapid clinical closure and healed with minimal evidence of the surgical procedure. The clinical outcome was also consistent with development of the desired ridge form. Cone-beam computed tomography imaging revealed an alveolar ridge form that was optimal for implant placement at 5 months post-treatment. The radiographic density of the augmented region was similar to the adjacent native bone, with no discernable interface between native and apparent new bone, consistent with integration and remodeling. Surgical re-entry for implant placement revealed the optimal ridge form (Figure 6 ). The implant was allowed to integrate for 5 months prior to the healing abutment placement and interim restoration. The overcontoured soft tissues enabled surgical sculpting to achieve the desired contour for the restorative emergence. A highly acceptable esthetic result was achieved. With preservation of the marginal and papillary gingival contours (Figure 7 and Figure 8 ); the radiographic presentation was consistent with normal bone remodeling and implant integration.
Case 2
A 48-year-old woman presented for implant therapy to replace a fractured maxillary right central (Figure 9). The treatment plan included the option of an immediate dental implant; however, a deep labial alveolar concavity precluded immediate implant placement without bone augmentation. This future implant site presented the esthetic challenge of maintaining both the gingival architecture and the relative position of the mucogingival junction across multiple surgical procedures.
Alternatives included the elevation of a mucoperiosteal flap with mobilization and advancement to obtain primary closure. Because no alveolar dehiscence or fenestration defect was anticipated at the extraction site, a submarginal incision could be made to provide access for augmentation and preserve the gingival architecture.41,42 The latter surgical approach provides necessary access while helping protect the marginal gingival contours and esthetics.
Nonsurgical techniques for socket grafting also have been advocated for the management of such clinical situations. Such approaches to site preservation couple the use of grafts with socket barrier materials, such as the socket seal surgery,43 which uses a partial-thickness graft of palatal masticatory mucosa, and the Bio-Col technique,44 which uses an absorbable collagen dressing (CollaPlug®, Integra, www.integra-ls.com). In a 15-year retrospective analysis, Landsberg45 concluded that the socket seal procedure is technique-sensitive and indicated only when an extraction socket is relatively intact and without evident preexisting inflammation. This investigator performed a relatively limited number of cases (112) during this period, perhaps reflecting the infrequency of extraction sockets with adequately retained bony housing. The application of a bone-grafting matrix enhanced with rhPDGF-BB may enable more compromised extraction sockets to be treated using these surgical techniques.
In the present case, a submarginal incision was made at the mucogingival gingival junction, revealing the facial fenestration defect (Figure 10 ). The mucosa was elevated to access and graft both the socket and fenestration defect. Both sites were protected by a collagen membrane (Bio-Gide®), and sutured to close the submarginal incision (Figure 11 ). Medical cyanoacrylate was placed over the socket wound to stabilize the collagen membrane. Healing was uneventful, and the soft-tissue contours suggested preservation of the alveolar contours at the time of extraction. Five months after extraction and augmentation, the findings from a clinical postoperative radiograph demonstrated evidence of notable graft containment as reflected by the overall radiopacity of the extraction site, even coronal to the socket. The site was reentered for implant placement. Flap elevation revealed the dense bone ridge with complete incorporation of the particulate graft material. The osteotomy for implant placement was prepared with a trephine bur, and the resulting specimen was submitted for histologic evaluation. The histologic findings revealed evidence of robust osteogenesis throughout the bone core, including the coronal aspect supporting the soft tissue. New bone was found in close amalgamation with the FDBA-graft particles throughout the specimen. A dental implant was placed at the site in a single-stage surgery and allowed to heal for 5 months prior to the restoration. The esthetic outcome was highly acceptable, with preservation of the marginal and papillary gingiva contours (Figure 12 ). The radiographic findings showed consistent normal bone remodeling and implant integration (Figure 13 ).
Case 3
A 51-year-old man presented with a 12-mm probing depth on the mesiobuccal and distalbuccal surfaces secondary to a root fracture extending to the apex of the maxillary left canine (Figure 14 ). The tooth was extracted without flap elevation, and the socket and periradicular defect were thoroughly degranulated using magnification and fiber optic illumination. The socket and bony defect were grafted with an FDBA wetted with rhPDGF-BB (10 minutes). A resorbable collagen membrane was cut, adapted, and stabilized with cyanoacrylate over the extraction socket, as in the Bio-Col technique.44 Healing proceeded uneventfully with clinical closure and epithelialization of the extraction site after 21 days. At 5 months post-treatment, the radiographic findings were consistent with complete bone fill of the defect (Figure 15 ). The osteotomy for implant placement was prepared with a trephine bur. The core specimen was submitted for histologic evaluation; the findings revealed areas of intense osteogenesis with new bone trabeculae surrounding and interconnecting the graft particles (Figure 16 ). Histologic evidence showed new bone repopulating regions of graft particles (Figure 17).
Conclusion
Recombinant growth factor technology has increased the options for combinatorial approaches to reconstructive surgery. Graft matrices that are space maintaining and osteoconductive aid in preventing soft-tissue collapse and provide a scaffold for cellular migration and stabilization of the blood clot. Graft matrices, such as ß-TCP and bone allograft, can also serve as delivery devices for drugs and biologics, although the release kinetics can differ among scaffolds. The potent effect of rhPDGF-BB on both bone and soft-tissue healing expands the ability to manage esthetic implant cases. For cases in which bone preservation is required, the tissue contours can be maintained with noninvasive or minimally invasive protocols. For sites requiring hard- and soft-tissue augmentation, these procedures can be combined to reduce the number of surgical experiences for patients. Bone-grafting materials, such as a mineralized bone allograft, combined with PDGF-BB appear to stimulate more robust bone formation and rapid wound closure, enhancing the development and preservation of bony and gingival contours critical for achieving esthetic implant outcomes. Although highly favorable clinical outcomes have been achieved using PDGF-BB in combination with allogenic bone grafts, the off-label use of this growth factor in combination with scaffolds other than ß-TCP or for therapeutic indications other than periodontal defects must be based on firm scientific rationale and sound medical evidence.
The clinical goals of growth-factor enhanced therapy include less invasive surgical procedures with more robust and predictable treatment outcomes.45,46 Although autogenous grafts remain widely considered the gold standard for the correction of localized ridge deformities,47 constraints in the volume of available autogenous bone and morbidity associated with graft harvest often limit treatment recommendations and patient acceptance. The ability to achieve optimal and predictable implant site development without the use of autogenous grafts offers great advantage to the clinician and patient.
The clinical application of bone allografts for the development of extraction sites, lateral and vertical ridge augmentation, and maxillary sinus augmentation is well documented in implant therapy.47-49 FDBA and DFDBA are highly osteoconductive and biocompatible, as evidenced by histologic incorporation and amalgamation with new bone.50,51 Clinical evidence supports the use of allogenic grafts for ridge augmentation for dental implant placement;52,53 however, the extent of bone regeneration appears variable and dependent on factors such as graft form—particulate versus block—and defect location.54 The addition of growth factors, such as rhPDGF-BB, offers important potential to overcome some of the clinical limitations of allogenic bone grafts.31
The cases presented in this article illustrate the application of growth-factor enhanced grafts and highlight the favorable clinical results achieved with this therapeutic approach. Controlled clinical trials are necessary to establish the relative effectiveness of rhPDGF-BB combined with different mammalian scaffolds for alveolar augmentation.
Disclosure
Dr. Nevins has received grant/research support from Osteohealth.
References
1. Langer R, Vacanti JP. Tissue engineering. Science. 1993;260(5110): 920-926.
2. Murphy KG. Postoperative healing complications associated with Gore-Tex Periodontal Material. Part II. Effect of complications on regeneration. Int J Periodontics Restorative Dent. 1995;15(6):548-561.
3. Machtei EE. The effect of membrane exposure on the outcome of regenerative procedures in humans: a meta-analysis. J Periodontol. 2001;72(4):512-516.
4. Buser D, Brägger U, Lang NP, Nyman S. Regeneration and enlargement of jaw bone using guided tissue regeneration. Clin Oral Implants Res. 1990;1(1):22-32.
5. Simion M, Baldoni M, Rossi P, Zaffe D. A comparative study of the effectiveness of e-PTFE membranes with and without early exposure during the healing period. Int J Periodontics Restorative Dent. 1994;14(2):166-180.
6. Wisner-Lynch LA. From passive to active: will recombinant growth factor therapeutics revolutionize regeneration? Int J Periodontics Restorative Dent. 2006;26(5):409-411.
7. Aichelmann-Reidy ME, Reynolds MA. Predictability of clinical outcomes following regenerative therapy in intrabony defects. J Periodontol. 2008;79(3):387-393.
8. Reynolds MA, Aichelmann-Reidy ME, Branch-Mays GL, Gunsolley JC. The efficacy of bone replacement grafts in the treatment of periodontal osseous defects. A systematic review. Ann Periodontol. 2003;8(1):227-265.
9. Eingartner C, Coerper S, Fritz J, et al. Growth factors in distraction osteogenesis. Immuno-histological pattern of TGF-beta1 and IGF-I in human callus induced by distraction osteogenesis. Int Orthop. 1999;23(5):253-259.
10. Mustoe TA, Pierce GF, Thomason A, et al. Accelerated healing of incisional wounds in rats induced by transforming growth factor-beta. Science. 1987;237(4820):1333-1336.
11. Noda M, Camilliere JJ. In vivo stimulation of bone formation by transforming growth factor-beta. Endocrinology. 1989;124(6):2991-2994.
12. Lind M, Deleuran B, Thestrup-Pedersen K, et al. Chemotaxis of human osteoblasts. Effects of osteotropic growth factors. APMIS. 1995;103(2):140-146.
13. Giannobile WV, Hernandez RA, Finkelman RD, et al. Comparative effects of platelet-derived growth factor-BB and insulin-like growth factor-I, individually and in combination, on periodontal regeneration in Macaca fascicularis. J Periodontal Res. 1996;31(5):301-312.
14. Mott DA, Mailhot J, Cuenin MF, et al. Enhancement of osteoblast proliferation in vitro by selective enrichment of demineralized freeze-dried bone allograft with specific growth factors. J Oral Implantol. 2002;28(2):57-66.
15. Giannobile WV, Finkelman RD, Lynch SE. Comparison of canine and non-human primate animal models for periodontal regenerative therapy: results following a single administration of PDGF/IGF-I. J Periodontol. 1994;65(12):1158-1168.
16. Rutherford RB, Niekrash CE, Kennedy JE, Charette MF. Platelet-derived and insulin-like growth factors stimulate regeneration of periodontal attachment in monkeys. J Periodontal Res. 1992;27(4 pt 1):285-290.
17. Lynch SE, de Castilla GR, Williams RC, et al. The effects of short-term application of a combination of platelet-derived and insulin-like growth factors on periodontal wound healing. J Periodontol. 1991;62(7):458-467.
18. Lynch SE, Williams RC, Polson AM, et al. A combination of platelet-derived and insulin-like growth factors enhances periodontal regeneration. J Clin Periodontol. 1989;16(8):545-548.
19. Nevins M, Camelo M, Nevins ML, et al. Periodontal regeneration in humans using recombinant human platelet-derived growth factor-BB (rhPDGF-BB) and allogenic bone. J Periodontol. 2003;74(9):1282-1292.
20. Camelo M, Nevins ML, Schenk RK, et al. Periodontal regeneration in human Class II furcations using purified recombinant human platelet-derived growth factor-BB (rhPDGF-BB) with bone allograft. Int J Periodontics Restorative Dent. 2003;23(3):213-225.2005;63(12):1693-1707.
22. Fiorellini JP, Howell TH, Cochran D, et al. Randomized study evaluating recombinant human bone morphogenetic protein-2 for extraction socket augmentation. J Periodontol. 2005;76(4):605-613.
23. Nevins M, Giannobile WV, McGuire MK, et al. Platelet-derived growth factor stimulates bone fill and rate of attachment level gain: results of a large multicenter randomized controlled trial. J Periodontol. 2005;76(12):2205-2215.
24. Howell TH, Fiorellini JP, Paquette DW, et al. A phase I/II clinical trial to evaluate a combination of recombinant human platelet-derived growth factor-BB and recombinant human insulin-like growth factor-I in patients with periodontal disease. J Periodontol. 1997;68(12):1186-1193.
25. Hollinger JO, Hart CE, Hirsch SN, et al. Recombinant human platelet-derived growth factor: biology and clinical applications. J Bone Joint Surg Am. 2008;90(suppl 1):48-54.
26. Homsi J, Daud AI. Spectrum of activity and mechanism of action of VEGF/PDGF inhibitors. Cancer Control. 2007;14(3):285-294.
27. Matsuda N, Lin WL, Kumar NM, et al. Mitogenic, chemotactic, and synthetic responses of rat periodontal ligament fibroblastic cells to polypeptide growth factors in vitro. J Periodontol. 1992;63(6):515-525.
28. Oates TW, Rouse CA, Cochran DL. Mitogenic effects of growth factors on human periodontal ligament cells in vitro. J Periodontol. 1993;64(2):142-148.
29. Cooke JW, Sarment DP, Whitesman LA, et al. Effect of rhPDGF-BB delivery on mediators of periodontal wound repair. Tissue Eng. 2006;12(6):1441-1450.
30. Sarment DP, Cooke JW, Miller SE, et al. Effect of rhPDGF-BB on bone turnover during periodontal repair. J Clin Periodontol. 2006;33(2):135-140.
31. Boyan BD, Ranly DM, Schwartz Z. Use of growth factors to modify osteoinductivity of demineralized bone allografts: lessons for tissue engineering of bone. Dent Clin North Am. 2006;50(2):217-228.
32. Nevins M, Hanratty J, Lynch SE. Clinical results using recombinant human platelet-derived growth factor and mineralized freeze-dried bone allograft in periodontal defects. Int J Periodontics Restorative Dent. 2007;27(5):421-427.
33. Lynch SE, Buser D, Hernandez RA, et al. Effects of the platelet-derived growth factor/insulin-like growth factor-I combination on bone regeneration around titanium dental implants. Results of a pilot study in beagle dogs. J Periodontol. 1991;62(11):710-716.
34. Becker W, Lynch SE, Lekholm U, et al. A comparison of ePTFE membranes alone or in combination with platelet-derived growth factors and insulin-like growth factor-I or demineralized freeze-dried bone in promoting bone formation around immediate extraction socket implants. J Periodontol. 1992;63(11):929-940.
35. Simion M, Rocchietta I, Kim D, et al. Vertical ridge augmentation by means of deproteinized bovine bone block and recombinant human platelet-derived growth factor-BB: a histologic study in a dog model. Int J Periodontics Restorative Dent. 2006;26(5):415-423.
36. Simion M, Nevins M, Rocchietta I, et al. Vertical ridge augmentation using an equine block infused with recombinant human platelet-derived growth factor-BB: a histologic study in a canine model. Int J Periodontics Restorative Dent. 2009;29(3):245-255.
37. Nevins ML, Camelo M, Nevins M, et al. Minimally invasive alveolar ridge augmentation procedure (tunneling technique) using rhPDGF-BB in combination with three matrices: a case series. Int J Periodontics Restorative Dent. 2009;29(4):371-383.
38. Nevins ML, Camelo M, Schupbach P, et al. Human histologic evaluation of mineralized collagen bone substitute and recombinant platelet-derived growth factor-BB to create bone for implant placement in extraction socket defects at 4 and 6 months: a case series. Int J Periodontics Restorative Dent. 2009;29(2):129-139.
39. Langer B, Calagna LJ. The subepithelial connective tissue graft. A new approach to the enhancement of anterior cosmetics. Int J Periodontics Restorative Dent. 1982;2(2):22-33.
40. Langer B, Calagna L. The subepithelial connective tissue graft. J Prosthet Dent. 1980;44(4):363-367.
41. Evian CI, Al-Momani A, Rosenberg ES, Sanavi F. Therapeutic management for immediate implant placement in sites with periapical deficiencies where coronal bone is present: technique and case report. Int J Oral Maxillofac Implants. 2006;21(3):476-480.
42. Steigmann M, Wang HL. Esthetic buccal flap for correction of buccal fenestration defects during flapless immediate implant surgery. J Periodontol. 2006;77(3):517-522.
43. Landsberg CJ, Bichacho N. A modified surgical/prosthetic approach for optimal single implant supported crown. Part I—The socket seal surgery. Pract Periodontics Aesthet Dent. 1994;6(2):11-17.
44. Sclar AG. Preserving alveolar ridge anatomy following tooth removal in conjunction with immediate implant placement. The Bio-Col technique. Atlas Oral Maxillofac Surg Clin North Am. 1999;7(2):39-59.
45. Landsberg CJ. Implementing socket seal surgery as a socket preservation technique for pontic site development: surgical steps revisited—a report of two cases. J Periodontol. 2008;79(5):945-954.
46. Lynch SE, Wisner-Lynch L, Nevins M, Nevins ML. A new era in periodontal and periimplant regeneration: use of growth-factor enhanced matrices incorporating rhPDGF. Compend Contin Educ Dent. 2006;27(12):672-680.
47. McAllister BS, Haghighat K. Bone augmentation techniques. J Periodontol. 2007;78(3):377-396.
48. Wallace SS, Froum SJ. Effect of maxillary sinus augmentation on the survival of endosseous dental implants. A systematic review. Ann Periodontol. 2003;8(1):328-343.
49. Aghaloo TL, Moy PK. Which hard tissue augmentation techniques are the most successful in furnishing bony support for implant placement? Int J Oral Maxillofac Implants. 2007;22(suppl):49-70.
50. Piattelli A, Scarano A, Corigliano M, Piattelli M. Comparison of bone regeneration with the use of mineralized and demineralized freeze-dried bone allografts: a histological and histochemical study in man. Biomaterials. 1996;17(11):1127-1131.
51. Yukna RA, Vastardis S. Comparative evaluation of decalcified and non-decalcified freeze-dried bone allografts in rhesus monkeys. I. Histologic findings. J Periodontol. 2005;76(1):57-65.
52. Donos N, Mardas N, Chadha V. Clinical outcomes of implants following lateral bone augmentation: systematic assessment of available options (barrier membranes, bone grafts, split osteotomy). J Clin Periodontol. 2008;35(8 suppl):173-202.
53. Rocchietta I, Fontana F, Simion M. Clinical outcomes of vertical bone augmentation to enable dental implant placement: a systematic review. J Clin Periodontol. 2008;35(8 suppl):203-215.
54. Waasdorp JR, Reynolds MA. Allogeneic bone onlay grafts for alveolar ridge augmentation: a systematic review. Int J Oral Maxillofac Implants. 2010;25(3):525-531.
About the Authors
Marc L. Nevins, DMD, MMSc
Clinical Assistant Professor
Department of Oral Medicine
Infection and Immunity
Harvard School of Dental Medicine
Boston, Massachusetts
Mark A. Reynolds, DDS, PhD
Professor, Chair, Department of Periodontics
University of Maryland Dental School
Baltimore, Maryland