You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
Globally, infertility impacts approximately one in six people and can lead to significant social, psychological, and economic consequences.1 It has been well-established that chronic systemic inflammation can impact fertility in both men and women by affecting ovarian function, sperm quality, and implantation. Chronic inflammation has been further linked to conditions like endometriosis, polycystic ovary syndrome (PCOS), and premature ovarian failure.2 Due to the prevalence of infertility and the wide-ranging impacts it can have on patients’ health and quality of life, a thorough patient medical history is critical to providing comprehensive and safe care for dental patients. Dental healthcare professionals need to have an in-depth understanding of reported medical conditions and medications to enable assessment of the patient’s overall systemic health. While periodontal disease has been linked to more than 57 systemic diseases and conditions, the exact nature and mechanism of the associations are still under investigation.3 A frequently identified mechanism linking periodontal health and overall health is the common inflammatory burden between many of these diseases.4
Infertility is one of many conditions that has been associated with chronic inflammation. With infertility rates on the rise, both medical and dental providers can play a vital role in screening for reproductive conditions and infertility risk factors and aiding in their prevention. Further, the establishment of good oral health and hygiene habits may promote better overall health, including enhanced fertility and pregnancy outcomes. As more understanding is gained on how oral health impacts infertility and systemic health overall, healthcare providers can more effectively tailor treatments and prevention strategies to meet individual patient needs. The relationship between oral health and overall health, including fertility, further reinforces the importance of interprofessional collaboration among dental and medical providers.
Both medical and dental healthcare providers should emphasize the need for enhanced awareness of the relationship between periodontal disease and overall health. Screening for periodontal disease and periodontal risk factors by medical providers and subsequent referral for comprehensive assessment and treatment of periodontal conditions could provide avenues to enhance dental care delivery for at-risk individuals. Despite recommendations from the American College of Obstetricians and Gynecologists for women who are pregnant or seeking to become pregnant to receive dental examinations and treatment, 56% of women do not receive dental care in the perinatal period and most women (59%) receive no counseling about oral health during pregnancy.5 It is recommended that perinatal healthcare providers include health questionnaire inquiries about oral health and dental history as a part of medical assessments for patients who are pregnant or seeking to become pregnant (Table 1).6 In a complementary fashion, dental healthcare providers should query patients about family planning, family history of infertility, and reproductive health to allow for patient counseling and better interprofessional communication. Moreover, gathering additional medical history information related to reproductive health can inform oral health examinations and risk assessments related to periodontal health.
What Is Infertility?
The World Health Organization defines infertility as the inability to achieve pregnancy after 12 months or more of unprotected sex. Affecting both males and females, infertility can be further described as primary or secondary. Primary infertility describes infertility that occurs in an individual who has never been pregnant, while secondary infertility is an inability to become pregnant after a previous successful pregnancy.7 Male infertility is defined as the inability to start a pregnancy with a female partner over a 12-month time period.8
Globally, infertility rates in both females and males are rising, with recent data reporting that approximately 17% of individuals worldwide struggle with infertility.8 While the causes of male and female infertility are multifactorial, known risk indicators such as lifestyle, diet, environmental factors, systemic health, and age contribute to increasing fertility rates. Particularly in females, infertility is strongly influenced by age and lifestyle and is more prevalent in individuals aged 30 to 40 years compared to younger individuals. In women, infertility is often associated with dysfunction of the ovulatory process and disorders of the reproductive system, including abnormal cervical mucus production, endometriosis, endometrial adhesions, and/or anatomical anomalies affecting fallopian tubes or the uterus.9 Additionally, known chronic inflammatory conditions such as obesity, PCOS, endometriosis, and diabetes mellitus have been directly related to female infertility and also linked to male infertility.
Current treatments for managing infertility can involve identification and treatment of underlying etiologies, including improving systemic health through diet and exercise as well as assisted reproductive technologies. Research has shown that obese women are at higher risk for reproductive dysfunction and perinatal complication rates.9 Obesity is considered a hyperinflammatory disease and is related to insulin resistance and endocrine disorders. When insulin and leptin levels increase, such as in cases of obesity and dysglycemia, hyperandrogenism can result, which can present in some patients as PCOS.
The use of novel drugs for hyperglycemia and weight loss, including glucagon-like peptide-1 receptor antagonist (GLP-1ra) and gastric inhibitory polypeptide medications, has demonstrated improved fertility, menstrual regularity, and estrogen disruption in females. Such improvements have been associated with loss of at least 10% of baseline body weight.10 Other infertility treatments include medications that modify sex steroid hormones and assisted reproductive technologies (ARTs), including intrauterine insemination and in vitro fertilization.11 It should be noted that success rates of such ARTs are impacted by the presence of inflammatory systemic health conditions.12
Periodontal Disease Epidemiology and Disease Progression
Periodontal diseases, including gingivitis and periodontitis, are initiated by dysbiotic biofilm, and hard- and soft-tissue destruction occurs through activation of the host immuno-inflammatory response.13 The most severe form of periodontal disease, periodontitis, is characterized by inflammation and infection of the soft tissue and bone surrounding teeth leading to resorption of the supporting structures around teeth and, ultimately, tooth loss.14 Periodontitis is known to be related to other chronic inflammatory conditions, such as cardiovascular disease and diabetes mellitus, and it affects more than 42% of US adults over the age of 30.15
Gingivitis is a reversible inflammatory disease that is a necessary precursor to periodontitis. According to the American Academy of Periodontology/European Federation of Periodontology World Workshop on the Classification of Periodontal and Peri-implant Diseases and Conditions, gingivitis is a nonspecific inflammatory lesion caused by the interaction between dental plaque biofilm and the host.16 Inflammation in gingivitis is limited to the gingiva and does not extend to the periodontal attachment apparatus.13,16 With cessation of adequate oral hygiene, 100% of individuals are susceptible to the development of gingivitis.13 Gingivitis, however, is reversible through improved oral hygiene and meticulous plaque control.7
Once gingivitis is established, inflammation and tissue destruction in susceptible individuals may progress to periodontitis without treatment. As biofilm continues to collect on teeth, complexity and dysbiosis of oral biofilms increases and pathogenic organisms and their virulence factors elicit a host immune-inflammatory response.17 Gram-negative bacteria, such as Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis, and Prevotella intermedia, are key players in periodontitis disease progression and contain virulence factors that trigger the host immune response.18 The periodontal inflammatory response to oral dysbiosis results in release of pro-inflammatory cytokines (eg, interleukin [IL]-1β, tumor necrosis factor [TNF]-α, IL-6) and enzymes associated with tissue breakdown such as matrix metalloproteinases (eg, MMP-8), which then activate destruction of hard and soft tissues of the periodontium.18
While this biofilm-induced inflammation and tissue destruction occurs locally at the surrounding tissues around teeth, the subsequent inflammatory markers, pathogenic bacteria, and bacterial virulence factors may also enter the systemic circulation and influence overall systemic wellness. A common inflammatory burden from periodontitis and chronic inflammatory diseases may exacerbate both these systemic conditions and periodontal tissue destruction.19 Treatments for periodontal disease involve removal and/or alteration of dysbiotic biofilm, elimination of niches frequently colonized by pathogenic bacteria, and host modulation strategies to alter the immune response to bacteria and virulence factors (Table 2).20 Successful periodontal therapy results in a long-term decrease in pro-inflammatory biomarkers locally and systemically, and this reduced inflammatory burden may ameliorate risks for systemic inflammatory conditions and decrease systemic disease severity and/or progression.21
Periodontal Disease and Infertility
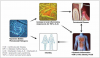
Patients with pre-existing periodontal disease have demonstrated decreased fertility rates and extended time to conception.22 Women with self-reported periodontitis have also reported higher levels of spontaneous abortion.23 Periodontitis and infertility have been linked through two mechanisms: (1) direct effects of pathogenic oral bacteria disseminated through the bloodstream on reproductive organs, and (2) the impact of pro-inflammatory cytokines and immunoglobulins on hormone levels and/or fertilization and implantation of embryos.19,24 A mechanism that implicates direct bacterial effects is supported by the identification of P gingivalis and Fusobacterium nucleatum in amniotic fluid or placental tissues in mothers with periodontitis who had premature births and/or stillbirths. Further, the presence of P gingivalis and A actinomycetemcomitans in the amniotic fluid of pregnant women with periodontal disease highlights the potential role of bacteria and bacterial products (Figure 1).19
Studies also suggest that female sex steroid hormones, particularly estradiol, are associated with alterations in the oral microbiome, particularly in premenopausal women.25 As estradiol is associated with reproductive health and elevated levels are linked to PCOS, this may indicate a bidirectional link between reproductive and periodontal health. Although studies are limited, there is evidence that illustrates a positive correlation of neutrophil–lymphocyte ratio and platelet–lymphocyte ratio, which often serve as markers of systemic inflammation and are often associated with periodontal disease severity, to infertility.26
An association has been identified between periodontal disease and infertility, and this link has attributed, in part, with a common inflammatory burden impacting both diseases. While this association does not imply causation, an increased prevalence of primary and secondary infertility was seen in individuals with periodontal disease compared to healthy controls. Further, it is well-established that active periodontitis has been associated with increased systemic inflammatory burden, insulin resistance, dysglycemia, and oxidative stress.27,28
PCOS has been associated with periodontitis prevalence29 and is also a well-established risk factor for infertility.30 Patients with PCOS are at higher risk for multiple comorbidities, including insulin resistance, diabetes mellitus type 2, cardiovascular diseases, obesity, malignancy, and psychosocial disorders.31 PCOS is often linked with chronic systemic inflammation, including increased pro-inflammatory cytokines.32 Individuals with PCOS demonstrate increased serum TNF-α levels, which has been linked to insulin resistance. Elevated TNF-α levels both locally in the gingival crevicular fluid and systemically are noted in patients with chronic inflammatory diseases, including diabetes mellitus and periodontitis. Elevated pro-inflammatory cytokines can result in delayed wound healing and enhanced periodontal disease progression and destruction of the periodontium.27 Conversely, periodontal disease progression results in elevated cytokine levels that can impact reproductive health.
In males, periodontitis has also been associated with poorer infertility outcomes. A statistically significant association has been demonstrated between periodontitis and erectile dysfunction with an odds ratio ranging from 1.53 to 5.94.33,34 Although the exact mechanisms are unclear, inflammation related to periodontal disease can influence endocrine function, including hormone levels. Further, the relationship between periodontitis and endothelial dysfunction may also impact blood flow during erection and result in reduced erectile function.35,36 If erectile dysfunction is determined to be vasculogenic, this may indicate an increased likelihood of association with periodontitis. There is also evidence to suggest that prevalence of periodontitis in men is associated with sperm and sperm-motility abnormalities.37
Clinical Implications for Patients With Periodontitis and Infertility
Studies indicate the association between periodontitis and infertility. By controlling modifiable risk factors, such as obesity and dysglycemia, patients may see improvements in overall health and decrease systemic inflammation, which may have a positive impact on both periodontal and reproductive outcomes. Individuals are also advised to seek regular dental care and present for periodontal therapy and maintenance to control the localized oral inflammatory burden, which will ultimately improve systemic inflammation as well. It has also been suggested that establishing periodontal health and a commensal oral microbiome prior to pregnancy could improve fecundity and reduce adverse pregnancy outcomes.38,39
Interprofessional care, communication, and bidirectional referral can also allow for more aligned care and enhanced periodontal and reproductive health. Healthcare providers addressing infertility and/or hormone disturbances should evaluate patients’ periodontal conditions through self-reported questionnaires and/or assessment of recent comprehensive oral examination.40 Patients without a dental home or recent dental visits should be encouraged to seek examination and treatment for periodontal diseases and oral sources of inflammation.
All healthcare providers, including dental care professionals, should advocate for whole-person healthcare and treat patients with improved systemic health as the main goal of treatment. Through collaboration among primary care physicians, perinatal healthcare providers, and dental healthcare professionals, patients seeking to conceive and those struggling with infertility can be informed of the potential role of oral inflammatory conditions, including periodontitis, on reproductive health and be referred for examination and treatment of periodontal diseases. Better understanding of the interactions between oral and systemic inflammation and the influence of oral inflammatory conditions on reproductive health can allow for implementation of interventions and policy changes that can positively impact fecundity and reproductive outcomes for patients.
Conclusion
With infertility prevalence increasing globally, it is important to identify underlying conditions that may contribute to the development or worsening of reproductive dysfunction, including suboptimal oral health and periodontal diseases. Chronic inflammatory conditions, such as cardiovascular disease, diabetes, obesity, and rheumatoid arthritis, play a role in the etiology of periodontal disease and have also demonstrated an association with poor reproductive health and infertility rates. Healthcare providers should recommend that individuals who are experiencing infertility seek care with a dental healthcare provider to screen for oral disease and coordinate ongoing care with medical professionals prior to conception and throughout the perinatal period.
ABOUT THE AUTHORS
Kallie Krueger, DMD
Second-Year (PGY2) Periodontal Resident, University of
Alabama at Birmingham School of Dentistry, Birmingham, Alabama
Maninder Kaur, BDS, MPH, MS
Associate Professor, Department of Periodontology,
University of Alabama at Birmingham School of Dentistry, Birmingham, Alabama;
Diplomate, American Board of Periodontology
Maria L. Geisinger, DDS, MS
Professor, Acting Chair, Director, Advanced Education
Program in Periodontology, Department of Periodontology, University of Alabama
at Birmingham School of Dentistry, Birmingham, Alabama; Diplomate, American
Board of Periodontology
Queries to the author regarding this course may be submitted to authorqueries@conexiant.com.
REFERENCES
1. Njagi P, Groot W, Arsenijevic J, et al. Financial costs of assisted reproductive technology for patients in low- and middle-income countries: a systematic review. Hum Reprod Open. 2023;2023(2):hoad007.
2. Weiss G, Goldsmith LT, Taylor RN, et al. Inflammation in reproductive disorders. Reprod Sci. 2009;16(2):216-229.
3. Huang D, Wang YY, Li BH, et al. Association between periodontal disease and systemic diseases: a cross-sectional analysis of current evidence. Mil Med Res. 2024;11(1):74.
4. Martínez-García M, Hernández-Lemus E. Periodontal inflammation and systemic diseases: an overview. Front Physiol. 2021;12:709438.
5. Hwang SS, Smith VC, McCormick MC, Barfield WD. Racial/ethnic disparities in maternal oral health experiences in 10 states, pregnancy risk assessment monitoring system, 2004-2006. Matern Child Health J. 2011;15(6):722-729.
6. Consortium for Oral Health Systems Integration and Improvement. Oral Health Risk Assessment Questions to Ask Pregnant Women. https://www.mchoralhealth.org/PDFs/compilation-oral-health-risk-assessment-questions-pregnant-women.pdf. Accessed August 27, 2025.
7. Zegers-Hochschild F, Adamson GD, Dyer S, et al. The
international
glossary on infertility and fertility care, 2017. Fertil Steril. 2017;108(3):393-406.
8. Márquez-Arrico CF, Silvestre FJ, Fernández-Reyes M, et al. Is there an association between periodontal disease and infertility? A systematic review. Med Oral Patol Oral Cir Bucal. 2024;29(6):e866-e875.
9. Dağ ZO, Dilbaz B. Impact of obesity on infertility in women. J Turk Ger Gynecol Assoc. 2015;16(2):111-117.
10. Jensterle M, Janez A, Fliers E, et al. The role of glucagon-like peptide-1 in reproduction: from physiology to therapeutic perspective. Hum Reprod Update. 2019;25(4):504-517.
11. Centers for Disease Control and Prevention. Reproductive Health. Infertility: frequently asked questions. CDC website. May 15, 2024. https://www.cdc.gov/reproductive-health/infertility-faq/index.html. Accessed August 26, 2025.
12. Rhodes TL, McCoy TP, Higdon HL 3rd, Boone WR. Factors affecting assisted reproductive technology (ART) pregnancy rates: a multivariate analysis. J Assist Reprod Genet. 2005;22(9-10):335-346.
13. Löe H, Theilade E, Jensen SB. Experimental gingivitis in man. J Periodontol. 1965;36:177-187.
14. American Academy of Periodontology. Glossary, Periodontitis. AAP website. October 6, 2012. https://members.perio.org/browse/glossary/search?executeSearch=true&ProductList=Announcement%2cBlog%2cCommunity%2cEgroup%2cQuestion%2cAnswer%2cCalendarEvent%2cGlossary%2cNavigation%2cLibrary%2cLibraryEntry&SearchTerm=Periodontitis&SearchMatch=all. Accessed August 26, 2025.
15. Eke PI, Thornton-Evans GO, Wei L, et al. Periodontitis in US adults: National Health and Nutrition Examination Survey 2009-2014. J Am Dent Assoc. 2018;149(7):576-588.e6.
16. Caton JG, Armitage G, Berglundh T, et al. A new classification scheme for periodontal and peri-implant diseases and conditions – introduction and key changes from the 1999 classification. J Periodontol. 2018;89 suppl 1:S1-S8.
17. Hajishengallis G, Chavakis T, Lambris JD. Current understanding of periodontal disease pathogenesis and targets for host-modulation therapy. Periodontol 2000. 2020;84(1):14-34.
18. Gasner NS, Schure RS. Periodontal disease. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; May 12, 2025.
19. Martelli ML, Brandi ML, Martelli M, et al. Periodontal disease and women’s health. Curr Med Res Opin. 2017;33(6):1005-1015.
20. Löe H, Anerud A, Boysen H, Morrison E. Natural history of periodontal disease in man. Rapid, moderate and no loss of attachment in Sri Lankan laborers 14 to 46 years of age. J Clin Periodontol. 1986;13(5):431-445.
21. D’Aiuto F, Orlandi M, Gunsolley JC. Evidence that periodontal treatment improves biomarkers and CVD outcomes. J Clin Periodontol. 2013;40 suppl 14:S85-S105.
22. Bond JC, Wise LA, Willis SK, et al. Self-reported periodontitis and fecundability in a population of pregnancy planners. Hum Reprod. 2021;36(8):2298-2308.
23. Bond JC, Wise LA, Fox MP, et al. Preconception periodontitis and risk of spontaneous abortion in a prospective cohort study. Am J Epidemiol. 2023;192(9):1509-1521.
24. Khanna SS, Dhaimade PA, Malhotra S. Oral health status and fertility treatment including IVF. J Obstet Gynaecol India. 2017;67(6):400-404.
25. Yakar N, Yilmaz B, Emingil G, et al. Subgingival microbial profiles in pre- and postmenopausal women: associations with serum estradiol levels. J Periodontol. 2025;96(1):97-108.
26. Duan Y, Zhou Y, Peng Y, et al. Inflammatory markers in women with infertility: a cross-sectional study. Int J Gen Med. 2023;16:1113-1121.
27. D’Aiuto F, Gkranias N, Bhowruth D, et al. Systemic effects of periodontitis treatment in patients with type 2 diabetes: a 12 month, single-centre, investigator-masked, randomised trial. Lancet Diabetes Endocrinol. 2018;6(12):954-965.
28. Mealey BL. Periodontal disease and diabetes. A two-way street. J Am Dent Assoc. 2006;137 suppl:26S-31S.
29. Rathi N, Reche A. Risk of periodontal diseases in women with polycystic ovary syndrome: an overview. Cureus. 2023;15(10):e47169.
30. Zhuang S, Jing C, Yu L, et al. The relationship between polycystic ovary syndrome and infertility: a bibliometric analysis. Ann Transl Med. 2022;10(6):318.
31. Tanguturi SC, Nagarakanti S. Polycystic ovary syndrome and periodontal disease: underlying links – a review. Indian J Endocrinol Metab. 2018;22(2):267-273.
32. Fuertes-Martín R, Moncayo S, Insenser M, et al. Glycoprotein A and B height-to-width ratios as obesity-independent novel biomarkers of low-grade chronic inflammation in women with polycystic ovary syndrome (PCOS). J Proteome Res. 2019;18(11):4038-4045.
33. Lecaplain B, Badran Z, Soueidan A, et al. Periodontitis, erectile dysfunction, reproductive hormones, and semen quality: a systematic review. Andrology. 2021;9(3):769-780.
34. Bizzarro S, Loos BG. The link between periodontitis and erectile dysfunction: a review. Br Dent J. 2019;227(7):599-603.
35. Orlandi M, Suvan J, Petrie A, et al. Association between periodontal disease and its treatment, flow-mediated dilatation and carotid intima-media thickness: a systematic review and meta-analysis. Atherosclerosis. 2014;236(1):39-46.
36. Piconi S, Trabattoni D, Luraghi C, et al. Treatment of periodontal disease results in improvements in endothelial dysfunction and reduction of the carotid intima-media thickness. Faseb J. 2009;23(4):1196-1204.
37. Tao DY, Zhu JL, Xie CY, et al. Relationship between periodontal disease and male infertility: a case-control study. Oral Dis. 2021;27(3):624-631.
38. Bond JC, Wise LA, Willis SK, et al. Self-reported periodontitis and fecundability in a population of pregnancy planners. Hum Reprod. 2021;36(8):2298-2308.
39. Ricci E, Ciccarelli S, Agnese Mauri P, et al. Periodontitis, female fertility and conception (review). Biomed Rep. 2022;17(5):86.
40. Blicher B, Joshipura K, Eke P. Validation of self-reported periodontal disease: a systematic review. J Dent Res. 2005;84(10):881-890.