You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
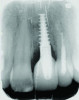
Retrograde peri-implantitis (RPI) was first described by McAllister et al in 1992 as a periapical lesion that develops after implant placement while the coronal portion of the implant retains a normal bone-to-implant interface.1,2 Clinical presentation of RPI may include pain, tenderness, redness, swelling, and, occasionally, fistula formation. Radiographically, RPI typically has a radiolucent area near the implant apex, signaling localized bone loss.3
The two forms of RPI are active and inactive. Active RPI manifests as symptomatic periapical radiolucency with an increase in lesion size, whereas inactive RPI presents as asymptomatic periapical radiolucency with either a stable or reduced lesion size, or an overextension of the implant osteotomy.4 The prevalence of RPI is relatively low (0.26% to 0.34%).5,6 Etiologic factors associated with RPI include endodontic lesions on adjacent teeth, bacterial contamination during implant placement, residual infection at the extraction site, excessive heat generation at the time of implant surgery, bone compression during surgery, overextension of osteotomy preparation, and systemic health conditions.7,8
In one investigation, retrograde peri-implantitis was shown to be more likely to occur the shorter the distance there was between the implant and endodontically treated (ie, root canal therapy [RCT]) tooth.9 RPI was significantly linked to time between implant placement and RCT on teeth exhibiting a periapical lesion. This relationship was also noted for distance between adjacent teeth and implants.9 To reduce the risk of RPI, a minimum distance of 2 mm between the implant and adjacent teeth with RCT is recommended.9 Additionally, delaying implant placement by at least 4 weeks after RCT is suggested.9 Microbial involvement plays a critical role in the pathogenesis of RPI with Porphyromonas gingivalis, Prevotella intermedia, Corynebacterium, Streptococcus, and Klebsiella pneumoniae being the most prevalent microorganisms.10-12
RPI management hinges on accurate etiologic diagnosis. Management involves surgical approaches, including mechanical curettage of the bony defect and chemical cleaning to detoxify the implant surface.13 When determining the choice of treatment, factors of the specific case need to be considered, including the severity of the infection, the amount of bone loss, and the patient’s overall health. Depending on the situation, a combination of treatments may be utilized to obtain the optimal outcome.
Studies have reported predictable outcomes using guided bone regeneration (GBR) to replace lost bone and stabilize the implant in cases of RPI.13-15 Most studies reported good implant survival (73.2% to 97.4%) 1 to 10 years following treatment.16-19 Another study reported cumulative implant survival rates of 78.3% over 20 years.14 Conversely, other studies reported that 35.7% of implants with RPI were removed, with significantly higher removal rates observed when adjacent teeth exhibited endodontic pathology.20
The current clinical series highlights two distinctly different appearances of RPI with GBR used as the selected treatment modality with follow-up of 1 to 7 years.
Materials and Methods
Clinical Presentations
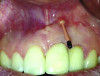
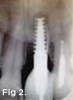
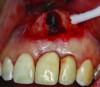
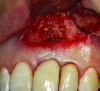
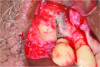
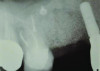
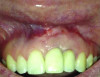
Patient 1: A 30-year-old female patient with an unremarkable medical history presented with implant No. 9 exhibiting retrograde peri-implantitis. Tooth No. 9 had been replaced with the implant 8 years prior because of a history of trauma and failed root canal treatment. The implant exhibited a periapical radiolucency with the sinus tract tracing to the apex of implant No. 9 (Figure 1 and Figure 2). The patient had a high smile line with longer clinical crowns at Nos. 9 and 10 compared with Nos. 7 and 8 (Figure 1 and Figure 2).
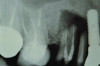
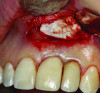
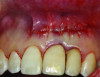
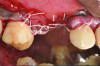
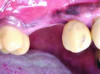
Patient 2: A 63-year-old healthy male patient presented with implant No. 6 exhibiting RPI, having been affected by previously endodontically treated and fractured tooth No. 5 (Figure 3 and Figure 4). The implant had probing depths ranging from 4 mm to 10 mm (Table 1) with the most severe bone loss at the distal aspect of implant No. 6 (Figure 3 and Figure 4).
Surgical Procedure
Treatment options were discussed with the patients, and both of them consented to the treatment of peri-implant debridement and GBR. Each patient received 600 mg of nonsteroidal anti-inflammatory drugs 1 hour before the procedure and pre-rinsed with 15 mL of 0.12% chlorhexidine gluconate; local anesthesia was administered using 2% lidocaine HCl with 1:100,000 epinephrine. Based on the presentation of the lesions, the incision designs and access differed for the two cases.
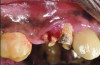
In case 1, due to the high smile line and asymmetrical clinical crown lengths, the patient received a vestibular incision at the mucogingival junction with full-thickness flap elevation to access the implant apex. Implant debridement was performed with titanium curettes and brushes (Figure 5). The implant surface was treated for 2 minutes with a doxycycline–saline slurry. The peri-implant defect was grafted using particulate freeze-dried bone allograft (FDBA) (Figure 6) and covered with a collagen membrane (Figure 7). Primary flap closure was obtained using resorbable sutures (Figure 8).
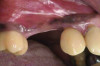
The patient in case 2 received a crestal incision with full-thickness flap elevation, then tooth No. 5 was extracted (Figure 9). Implant debridement followed the same protocol used in case 1. FDBA was mixed with a small amount of doxycycline and placed around the implant (Figure 10). A resorbable collagen membrane (not pictured) was secured with a tack. Primary closure was achieved with expanded polytetrafluoroethylene (ePTFE) sutures (Figure 11). An immediate post-surgical radiograph showed bone fill and the tack (Figure 12).
Post-surgically, both patients were prescribed amoxicillin 500 mg and ibuprofen 800 mg three times daily for 7 days along with chlorhexidine. Post-surgical instructions were provided, and the patients were to resume gentle brushing after 4 weeks. They received periodontal maintenance every 3 months and were followed postoperatively for 12 months (case 1) and 1 to 7 years (case 2).
Results
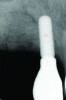
Healing was uneventful in both cases with no adverse outcomes or complications. Case 1 showed good healing with resolution of the sinus tract at 3 months post-surgically. Radiographs at 6- and 9-month follow-ups demonstrated increased bone opacity with complete radiographic bone fill at 12 months (Figure 13 and Figure 14).
The 12-month and 7-year follow-ups for case 2 showed similar improvements with normal soft-tissue healing and bone fill and normal pocket depths at 12 months (Figure 15 and Figure 16) and 7 years post-treatment (Figure 17 and Figure 18) (Table 1).
Discussion
Retrograde peri-implantitis presents a complex clinical challenge. Various treatments, including surgical debridement, apicoectomy, and GBR, have been proposed.5,20 Regrettably, efficacy of these interventions remains unclear due to limited clinical evidence supporting standardized treatment protocols.6 Despite a lack of definitive evidence on efficacy, surgical therapy using GBR is generally preferred.14 One systematic review reported significant improvements in implant stability and bone fill with GBR,16 and long-term implant survival rates have been reported.6
In the present case series, both patients showed successful resolution of RPI, proper bone fill, and stable peri-implant tissues over 1 to 7 years of follow-up. Radiographic assessments demonstrated good bone fill and stable probing depths over time, supporting the efficacy of regenerative surgical approaches in RPI management. These findings are consistent with previous reports demonstrating favorable outcomes with GBR in peri-implant defect management.14
Microbiological evidence suggests that persistent bacterial infections, often from adjacent compromised teeth, play a key role in RPI development.10-12 Early intervention, including thorough site decontamination and antimicrobial therapy, may enhance treatment success.8 Ongoing research is focused on optimizing regenerative techniques and antimicrobial strategies to improve clinical outcomes.13 The use of novel biomaterials such as amnion-chorion membranes and bioactive bone substitutes has shown promise in promoting peri-implant defect healing.15 These materials possess anti-inflammatory and antibacterial properties, potentially reducing recurrence rates and enhancing regeneration.14
Future studies should aim to refine treatment protocols and establish evidence-based guidelines for RPI management.18 While current evidence supports surgical intervention as the most effective treatment, additional research is needed to promote predictable long-term success.2
Conclusion
Characterized by uncommon and often progressive bone loss at the periapex of a dental implant, RPI presents a difficult clinical challenge. Various treatment modalities have been proposed, including surgical debridement, apicoectomy, and GBR. While current evidence supports surgical intervention as the most effective treatment approach, the outcomes should be interpreted cautiously due to limited long-term studies, variability in treatment protocols, and a lack of standardized diagnostic criteria. Further studies are necessary to establish a more predictable and evidence-based approach for managing RPI.
ABOUT THE AUTHORS
Anthony L. Neely, DDS, M.Dent.Sc., PhD
Associate Professor, Department of Graduate Periodontics, Division of Graduate Education, University of Detroit Mercy School of Dentistry, Detroit, Michigan; Private Practice, Southfield, Michigan
Minkie Kim, DDS
Periodontal Resident, Department of Graduate Periodontics, Division of Graduate Education, University of Detroit Mercy School of Dentistry, Detroit, Michigan
Joseph Samona, DDS
Periodontal Resident, Department of Graduate Periodontics, Division of Graduate Education, University of Detroit Mercy School of Dentistry, Detroit, Michigan
Jung I. Yoon, DMD
Periodontal Resident, Department of Graduate Periodontics, Division of Graduate Education, University of Detroit Mercy School of Dentistry, Detroit, Michigan
Bassam M. Kinaia, DDS, MS
Associate Professor, Department of Graduate Periodontics, Division of Graduate Education, University of Detroit Mercy School of Dentistry, Detroit, Michigan; Private Practice, Sterling Heights, Michigan
Queries to the author regarding this course may be submitted to authorqueries@conexiant.com.
REFERENCES
1. McAllister BS, Masters D, Meffert RM. Treatment of implants demonstrating periapical radiolucencies. Pract Periodontics Aesthet Dent. 1992;4(9):37-41.
2. Quirynen M, Gijbels F, Jacobs R. An infected jawbone site compromising successful osseointegration. Periodontol 2000. 2003;33:129-144.
3. Solomonov M, Via S, Dinur N, et al. Retrograde peri-implantitis: incidence and possible co-existing factors: a retrospective analysis. Aust Dent J. 2022;67(4):340-343.
4. Reiser GM, Nevins M. The implant periapical lesion: etiology, prevention, and treatment. Compend Contin Educ Dent. 1995;16(8):768-772.
5. Quirynen M, Vogels R, Alsaadi G, et al. Predisposing conditions for retrograde peri-implantitis, and treatment suggestions. Clin Oral Implants Res. 2005;16(5):599-608.
6. Di Murro B, Corrente G, Abundo R, et al. Prevalence and treatment of retrograde peri-implantitis: a retrospective cohort study covering a 20-year period. Clin Oral Investig. 2021;25(7):4553-4561.
7. Chan HL, Wang HL, Bashutski JD, et al. Retrograde peri-implantitis: a case report introducing an approach to its management. J Periodontol. 2011;82(7):1080-1088.
8. Sarmast ND, Wang HH, Soldatos NK, et al. A novel treatment decision tree and literature review of retrograde peri-implantitis. J Periodontol. 2016;87(12):1458-1467.
9. Zhou W, Han C, Li D, et al. Endodontic treatment of teeth induces retrograde peri-implantitis. Clin Oral Implants Res. 2009;20(12):1326-1332.
10. Marshall G, Canullo L, Logan RM, Rossi-Fedele G. Histopathological and microbiological findings associated with retrograde peri-implantitis of extra-radicular endodontic origin: a systematic and critical review. Int J Oral Maxillofac Surg. 2019;48(11):1475-1484.
11. Gomes BP, Pinheiro ET, Gadê-Neto CR, et al. Microbiological examination of infected dental root canals. Oral Microbiol Immunol. 2004;19(2):71-76.
12. Nair PNR. On the causes of persistent apical periodontitis: a review. Int Endod J. 2006;39(4):249-281.
13. Dahlin C, Nikfarid H, Alsén B, Kashani H. Apical peri-implantitis: possible predisposing factors, case reports, and surgical treatment suggestions. Clin Implant Dent Relat Res. 2009;11(3):222-227.
14. Peñarrocha-Diago MA, Blaya-Tárraga JA, Menéndez-Nieto I, et al. Implant survival after surgical treatment of early apical peri-implantitis: an ambispective cohort study covering a 20-year period. Int J Oral Implantol (Berl). 2020;13(2):161-170.
15. Tözüm TF, Sençimen M, Ortakoğlu K, et al. Diagnosis and treatment of a large periapical implant lesion associated with adjacent natural tooth: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(6):e132-e138.
16. Peñarrocha-Diago M, Peñarrocha-Diago M, Blaya-Tárraga JA. State of the art and clinical recommendations in periapical implant lesions. 9th Mozo-Grau Ticare Conference in Quintanilla, Spain. J Clin Exp Dent. 2017;9(3):e471-e473.
17. Peñarrocha-Diago M, Boronat-Lopez A, García-Mira B. Inflammatory implant periapical lesion: etiology, diagnosis, and treatment – presentation of 7 cases. J Oral Maxillofac Surg. 2009;67(1):168-173.
18. Balshi SF, Wolfinger GJ, Balshi TJ. A retrospective evaluation of a treatment protocol for dental implant periapical lesions: long-term results of 39 implant apicoectomies. Int J Oral Maxillofac Implants. 2007;22(2):267-272.
19. Lefever D, Van Assche N, Temmerman A, et al. Aetiology, microbiology and therapy of periapical lesions around oral implants: a retrospective analysis. J Clin Periodontol. 2013;40(3):296-302.
20. Ramanauskaite A, Juodzbalys G, Tözüm TF. Apical/retrograde periimplantitis/implant periapical lesion: etiology, risk factors, and treatment options: a systematic review. Implant Dent. 2016;25(5):684-697.