You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
Maintaining proper oral hygiene is crucial for the health of peri-implant tissues and the overall success of dental implants. Clinicians should aim to provide implant treatment that minimizes patients’ discomfort during their daily home care routines. This approach can enhance patient compliance and contribute to better long-term outcomes for implant health. Thus, the importance of peri-implant tissue in maintaining implant health and stability has been a focal point of research and debate in recent years.
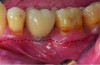
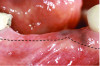
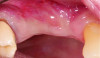
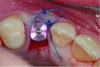
A deficient dimension of keratinized mucosa width (KMW) around dental implants is often related to patient discomfort during oral hygiene (Figure 1). Literature suggests the dimension of KMW should be considered as a risk factor for peri-implant mucositis and peri-implantitis.1-4 KMW is also associated with patient satisfaction regarding implant esthetics. Mucosal thickness (MT) is crucial for both esthetic outcomes and peri-implant health.5-7 Thicker mucosal tissue provides stability for the mucosal margin, which leads to less risk of mucosal recession. This underscores the importance of considering tissue thickness in implant planning and restoration.
This article is intended to aid clinicians in identifying the soft-tissue components in implant therapy at the diagnosis and treatment planning phase and during the treatment with an emphasis on the healed edentulous ridge for a staged implant placement approach. Clinical scenarios are presented with treatment options for soft-tissue enhancement.
Soft-Tissue Management
As emerging evidence shows that peri-implant mucosa is a risk factor for dental implant health, it is important to understand the specific aspects of the peri-implant mucosa. The clinical presentation of peri-implant tissue consists of KMW and MT, the two components of the peri-implant phenotype.8 While periodontal phenotype around natural teeth is assigned to an individual patient, peri-implant phenotype is determined by the condition of the future implant site and how that area is prepared and treated by the clinician, who has access to both bone and soft tissue throughout all steps of the implant therapy.
Assessing the Amount of KMW and MT
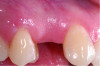
KMW is the dimension of keratinized soft tissue extending from the mucosal margin to the mucogingival junction (MGJ) in the apicocoronal direction. KMW is the coronal component of the peri-implant soft tissues, and beyond these tissues apically is the non-keratinized alveolar mucosa (Figure 1 through Figure 3). Where KMW is absent in certain conditions, there is solely non-keratinized peri-implant mucosa adaptation to the implant prosthesis. KMW is measured clinically with an instrument such as a periodontal probe in the apicocoronal direction, or an estimate of KMW can be made based on photographs. While as a clinical guideline or for research purposes it has been proposed that at least 2 mm of KMW be present, this amount is still debated as to whether or not it is necessary.8
MT is the horizontal dimension of the peri-implant soft tissue and may or may not be keratinized. MT is an important aspect of implant esthetic outcomes, especially at the most coronal aspect around the implant restoration. Around thin mucosa the color of the underlying restoration material may show through and result in esthetic deficiencies (Figure 1). Conversely, thick tissue can minimize the shading effect of underlying restoration materials and may compensate for any bony dehiscence around the implant body. In a systematic review, it was reported that after soft-tissue augmentation was conducted to increase MT, less interproximal marginal bone loss resulted around the implant.9
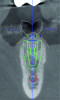
MT can be measured directly after a surgical flap elevation with an instrument such as a ruler or indirectly during a treatment planning stage with a digital measuring tool in an implant treatment planning software program (Figure 4 and Figure 5). As with KMW, it has been proposed that at least 2 mm of MT be present, although a lack of literature exists regarding its minimum dimension.8
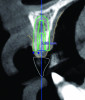
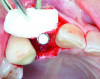
An example of an implant treatment planning case is presented in Figure 2 and Figure 4, with Figure 2 showing a digital scan of a mandibular arch that includes a missing tooth No. 19. The scan shows there was adequate KMW (>2 mm) at the No. 19 area. The digital scan of No. 19 was superimposed with a cone-beam computed tomography (CBCT) scan to analyze the future implant site. Upon superimposition, the MT could be measured at any surface (Figure 4). MT at the direct buccal and direct lingual aspects of the peri-implant mucosa was measured at >2 mm. The simulated future implant placement and the outlined safety zone of 1.5 mm radially and 2 mm apically around the implant indicate that the thickness of bone at the implant site was sufficient as planned. This case demonstrates there was enough KMW, bone thickness, and MT to proceed with implant placement.
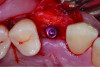
Another example of an implant treatment planning case, on a missing tooth No. 7, is shown in Figure 3 and Figure 5. There was adequate KMW (>2 mm) in the area of the missing tooth, but a concave contour was present on the buccal aspect of the ridge at the crest (Figure 3). A digital tooth set-up for the missing tooth No. 7 was superimposed with a CBCT. Upon superimposition, the MT could be measured at any surface (Figure 5). The simulated future implant placement and the safety zone marked around the implant indicate the thickness of the bone at the implant site was sufficient as planned. The MT, however, was <2 mm with clinical presence of a lack of soft-tissue volume. This clinical example demonstrates the need for soft-tissue enhancement to increase MT.
Managing the Deficiency
With the essential role of soft tissue in implant dentistry receiving significant attention in recent years, clinical treatment options and timing of therapy for soft-tissue enhancement have garnered a great deal of consideration.10,11 Autogenous free gingival grafts (FGGs) or connective tissue grafts (CTGs) are commonly used in the management of soft-tissue deficiencies because of the satisfactory results shown around the natural dentition.12,13 Autogenous grafts have been reported to bring a predictable outcome for increasing KMW or MT.14,15 However, due to the need for a secondary surgical site for harvesting these grafts, pain and discomfort are associated with these procedures. Low patient satisfaction related to the esthetic results with this modality, including mismatch of color and surface configuration of the mucosa, has been reported.16
To circumvent patient morbidity and improve esthetic outcomes, soft-tissue alternatives have been developed for and evaluated around both natural teeth and implants. These alternatives are derived from either allogenic or xenogenic sources such as allogenic acellular dermal matrix (ADM), xenogeneic acellular dermal matrix (AXDM), or xenogeneic collagen matrix (CM).13,16-19
Timing of Soft-Tissue Management
The surgical choice for soft-tissue management and the timing of it depends on the individual patient’s needs and the specific circumstances of the case. The patient’s medical condition, past periodontal history, and current periodontal situation are principal factors to consider when selecting a soft-tissue management technique and determining when to execute it.
If the patient’s medical condition does not permit extensive surgical procedures or indicates reduced healing potential, a minimally invasive option should be explored with alternative techniques and materials (eg, ADM, AXDM, or CM). If a patient has a history of periodontitis, creating a good hygienic environment, including developing enough KMW and MT for future implant therapy, would be necessary. A comprehensive examination that includes past medical history, periodontal assessment, and measuring bone thickness/MT/KMW is required for treatment planning of the soft-tissue management. Moreover, in cases where the patient has high esthetic demands, soft-tissue management could be more extensive beyond obtaining the minimal dimensions.
When soft-tissue deficiency is noted, early intervention may be ideal to increase predictability, and, therefore, the best time for managing soft tissue would likely be prior to implant placement.10 A staged approach to perform ridge augmentation to increase alveolar ridge width and height prior to implant placement is a common practice for a predictable outcome. When ridge augmentation is planned, the need for soft-tissue enhancement should be reviewed and planned accordingly. The soft-tissue enhancement can be done simultaneously with bone augmentation if it is for MT, or it may be staged if it is for KMW.
At implant placement or upon healing abutment connection, the surgical intervention for soft-tissue enhancement could be done if the implant site allows the surgical placement without prior ridge augmentation. When good primary stability of the implant is obtained at surgical implant placement without an additional bone grafting procedure, healing abutments can be placed instead of cover screws. Often, the healing abutments are placed later for proper healing after implants are placed with cover screws. During surgical placement of the implant or at the time of healing abutment connection are both recommended times for soft-tissue management.
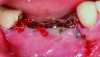
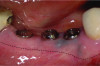
When a lack of KMW is noted, suggested treatments may include the use of an apically positioned flap (APF)20 (Figure 6 through Figure 8) or a combination of APF with an FGG (Figure 9 through Figure 11) or tissue alternatives.21 Because of the nature of the recipient site preparation for an FGG or tissue alternative, such as ADM, AXDM, or CM, there is a risk of wound dehiscence and a potential negative effect on the healing of the bone augmentation or on osseointegration if the grafting is done at the time of ridge augmentation or implant placement. Therefore, the preferred treatment time for increasing KMW with an FGG, ADM, AXDM, or CM is either prior to implant placement or after healing of the implant at the healing abutment connection (Table 1). MT can be increased using the roll technique (Figure 12 through Figure 14)22,23 or by adding a CTG (Figure 15 and Figure 16), ADM, AXDM, or CM (Figure 17 and Figure 18), either at the time of implant placement or healing abutment connection (Table 1).24,25
Discussion
The tissue around a dental implant greatly impacts peri-implant health and the esthetic outcome and is likely to influence the patient’s overall satisfaction with the implant restoration. When providing implant dentistry, clinicians should create hygiene-accessible peri-implant conditions while considering the patient’s need and desire for function and esthetics.
Recognizing risk factors for peri-implant health and the overall success of the implants during the diagnosis phase and before any treatment plan is completed is important. Patients can then make an educated decision with the information provided. In implant therapy, clinicians need to create a proper peri-implant mucosa phenotype within the limits of biology that favors long-lasting stability.
Because greater attention has been paid more recently to soft tissue related to peri-implant health, interest in how to manage soft tissue in implant dentistry has heightened. When soft-tissue deficiency is noted, early intervention would be ideal to increase predictability. There are three timepoints for clinicians to address soft-tissue management: prior to implant placement, at the time of implant placement, or at the time of the healing abutment connection. The selection of a surgical technique and its timing depends on the condition of the specific case and the goal of the treatment. The goal could be to increase either KMW, MT, or both. Studies that compared soft-tissue grafting procedures found that such procedures overall were beneficial for maintaining healthy peri-implant mucosa and bone around dental implants. After the procedure, KMW was increased and there was less bleeding at peri-implant mucosal sites and better marginal bone level. The thickened mucosa was associated with less marginal bone loss.9,14,17
Although various surgical techniques and materials are available for treating the soft-tissue around implants, research has not proven the superiority of any given method or material over others. Regardless, it is essential for clinicians to consider the tissue around the implant during treatment planning to achieve a predictable outcome. Because soft-tissue alternatives have shown comparable outcomes to autogenous grafts for soft-tissue enhancement while allowing for less morbidity and more comfort for patients, these alternative choices need to be considered as viable options and offered to patients when indicated.
The long-term effects of a lack of KMW and/or deficient MT need to be evaluated both clinically and scientifically in the future for the sake of evidence-based dentistry and to allow the best interests of patients’ oral health to be served.
Conclusion
Effective management of the peri-implant soft-tissue phenotype—particularly keratinized mucosa width and mucosal thickness—is essential for long-term implant success, patient comfort, and esthetic satisfaction. Clinicians must incorporate careful assessment and planning of soft-tissue dimensions at every stage of implant therapy to create stable, hygienic, and visually pleasing outcomes. While multiple techniques and materials exist for soft-tissue enhancement, no single method has proven superior; thus, personalized treatment based on individual patient needs and conditions remains key. Ongoing research and clinical evaluation will further refine soft-tissue protocols, ensuring the use of evidence-based strategies that enhance both implant longevity and patient-centered care.
ABOUT THE AUTHORS
Yoon Jeong Kim, DDS, MS
Professor, Director, Advanced Education in Periodontics, School of Dentistry, Loma Linda University, Loma Linda, California
Nabat Davrani, DDS
Third-Year Student in Advanced Education in Periodontics, School of Dentistry, Loma Linda University, Loma Linda, California
Derek Onjukka, DDS
Second-Year Student in Advanced Education in Periodontics, School of Dentistry, Loma Linda University, Loma Linda, California
Yekta Asadi, DDS
Second-Year Student in Advanced Education in Periodontics, School of Dentistry, Loma Linda University, Loma Linda, California
Queries to the author regarding this course may be submitted to authorqueries@conexiant.com.
REFERENCES
1. da Silva DM, Castro F, Martins B, et al. The influence of the gingival phenotype on implant survival rate and clinical parameters: a systematic review. Evid Based Dent. 2025. doi: 10.1038/s41432-025-01114-x.
2. Sabri H, Tavelli L, Sheikh AT, et al. Significance of peri-implant keratinised mucosa on implant health: an umbrella systematic review with evidence mapping and quantitative meta-meta-analysis. Int J Oral Implantol (Berl). 2025;18(1):13-30.
3. Perussolo J, Souza AB, Matarazzo F, et al. Influence of the keratinized mucosa on the stability of peri-implant tissues and brushing discomfort: a 4-year follow-up study. Clin Oral Implants Res. 2018;29(12):1177-1185.
4. Bonino F, Steffensen B, Natto Z, et al. Prospective study of the impact of peri-implant soft tissue properties on patient-reported and clinically assessed outcomes. J Periodontol. 2018;89(9):1025-1032.
5. Di Gianfilippo R, Valente NA, Toti P, et al. Influence of implant mucosal thickness on early bone loss: a systematic review with meta-analysis. J Periodontal Implant Sci. 2020;50(4):209-225.
6. Suarez-Lopez Del Amo F, Lin GH, Monje A, et al. Influence of soft tissue thickness on peri-implant marginal bone loss: a systematic review and meta-analysis. J Periodontol. 2016;87(6):690-699.
7. Wang IC, Barootchi S, Tavelli L, Wang HL. The peri-implant phenotype and implant esthetic complications. Contemporary overview. J Esthet Restor Dent. 2021;33(1):212-223.
8. Avila-Ortiz G, Gonzalez-Martin O, Couso-Queiruga E, Wang HL. The peri-implant phenotype. J Periodontol. 2020;91(3):283-288.
9. Thoma DS, Naenni N, Figuero E, et al. Effects of soft tissue augmentation procedures on peri-implant health or disease: a systematic review and meta-analysis. Clin Oral Implants Res. 2018;29(suppl 15):32-49.
10. Thoma DS, Gil A, Hämmerle CHF, Jung RE. Management and prevention of soft tissue complications in implant dentistry. Periodontol 2000. 2022;88(1):116-129.
11. Monje A, González-Martín O, Ávila-Ortiz G. Impact of peri-implant soft tissue characteristics on health and esthetics. J Esthet Restor Dent. 2023;35(1):183-196.
12. Chambrone L, Botelho J, Machado V, et al. Does the subepithelial connective tissue graft in conjunction with a coronally advanced flap remain as the gold standard therapy for the treatment of single gingival recession defects? A systematic review and network meta-analysis. J Periodontol. 2022;93(9):1336-1352.
13. Carbone AC, Joly JC, Botelho J, et al. Long-term stability of gingival margin and periodontal soft-tissue phenotype achieved after mucogingival therapy: a systematic review. J Clin Periodontol. 2024;51(2):177-195.
14. Tavelli L, Barootchi S, Avila-Ortiz G, et al. Peri-implant soft tissue phenotype modification and its impact on peri-implant health: a systematic review and network meta-analysis. J Periodontol. 2021;92(1):21-44.
15. Moussa H, Nasri W, Gargouri R, Bouslema A. Management of soft tissue defects around single implants: a systematic review of the literature. Clin Exp Dent Res. 2024;10(6):e70003.
16. Thoma DS, Buranawat B, Hammerle CH, et al. Efficacy of soft tissue augmentation around dental implants and in partially edentulous areas: a systematic review. J Clin Periodontol. 2014;41(suppl 15):S77-S91.
17. Cairo F, Barbato L, Selvaggi F, et al. Surgical procedures for soft tissue augmentation at implant sites. A systematic review and meta-analysis of randomized controlled trials. Clin Implant Dent Relat Res. 2019;21(6):1262-1270.
18. De Annuntiis C, Testarelli L, Guarnieri R. Use of xenogenic collagen matrices in peri-implant soft tissue volume augmentation: a critical review on the current evidence and new technique presentation. Materials (Basel). 2022;15(11):3937.
19. Costa MSC, Daltro Rosa CDDR, Bento VAA, et al. Efficacy of acellular xenogeneic dermal matrix graft in the treatment of multiple gingival recessions: systematic review and meta-analysis. Clin Oral Investig. 2024;28(3):177.
20. Tunkel J, de Stavola L, Khoury F. Changes in soft tissue dimensions following three different techniques of stage-two surgery: a case series report. Int J Periodontics Restorative Dent. 2013;33(4):411-418.
21. Bassetti RG, Stähli A, Bassetti MA, Sculean A. Soft tissue augmentation procedures at second-stage surgery: a systematic review. Clin Oral Investig. 2016;20(7):1369-1387.
22. Scharf DR, Tarnow DP. Modified roll technique for localized alveolar ridge augmentation. Int J Periodontics Restorative Dent. 1992;12(5):415-425.
23. Park SH, Wang HL. Pouch roll technique for implant soft tissue augmentation: a variation of the modified roll technique. Int J Periodontics Restorative Dent. 2012;32(3):e116-e121.
24. Hosseini M, Worsaae N, Gotfredsen K. Tissue changes at implant sites in the anterior maxilla with and without connective tissue grafting: a five-year prospective study. Clin Oral Implants Res. 2020;31(1):18-28.
25. Huber S, Zeltner M, Hämmerle CHF, et al. Non-interventional 1-year follow-up study of peri-implant soft tissues following previous soft tissue augmentation and crown insertion in single-tooth gaps. J Clin Periodontol. 2018;45(4):504-512.