You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
Periodontal phenotype is defined by the physical expression of the periodontal tissues, including alveolar bone and gingiva. Periodontal phenotype is influenced by genetics but can be modified by environmental factors and clinical interventions.1
The importance of periodontal phenotype is highlighted by the response of thin and thick phenotypes to insults like physical trauma and gingival inflammation. Observation of long-term clinical outcomes at sites demonstrating thin versus thick periodontal phenotypes demonstrates significantly more stability of gingival margin position at sites with thick phenotype.1 Sites with thin periodontal phenotype generally respond to trauma or inflammation with gingival recession and loss of alveolar bone height, whereas sites with thick periodontal phenotype are more resistant to damage from trauma and may respond to inflammation with pocket and intrabony defect formation.2,3 Studies suggest that at sites with thin periodontal phenotype, existing gingival recession defects will progress and new gingival recession defects will form if phenotype modification is not performed, while gingival recession at thick periodontal phenotype tends to be self-limiting, particularly if causative factors are eliminated.4,5 In essence, a thin periodontal phenotype is more vulnerable to loss of attachment after insult, which can lead to complications of periodontal health and stability, esthetics, and more.
Definitions
Phenotype describes the observable characteristics of an organism resulting from the interplay of genetic and environmental factors. In contrast, genotype or biotype refers to genetically predetermined characteristics, which are not modifiable. Thus, "periodontal biotype" is used to describe the natural condition of an individual's periodontium without any environmental changes, whereas "periodontal phenotype" describes the current condition resulting from a combination of the biotype and results of environmental factors such as trauma or surgical interventions.1,6,7
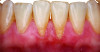
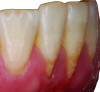
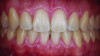
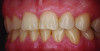
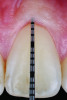
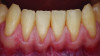
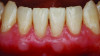
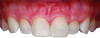
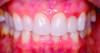
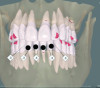
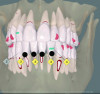
Periodontal phenotype is specifically defined as the combination of gingival phenotype (3-dimensional gingival volume, including keratinized tissue width and gingival thickness) and the alveolar bone morphotype (thickness of the bony plates),1 with later definitions also including tooth dimension.7 Zweers and colleagues described three groups of periodontal biotype (now referred to as periodontal phenotype): thin scalloped, thick scalloped, and thick flat, although the authors also state that up to one-third of the population presents with a mixed phenotypic presentation. The thin scalloped group is classified as having slender triangular-shaped crowns, subtle cervical convexity, interproximal contacts close to the incisal edge, a narrow zone of keratinized tissue, transparent gingiva, and relatively thin alveolar bone (Figure 1 and Figure 2). Thick scalloped phenotype exhibits thick and fibrotic gingiva, slender teeth, a narrow zone of keratinized tissue, and high gingival scallop (Figure 3). Lastly, the thick flat phenotype is associated with square-shaped crowns, pronounced cervical convexities, longer interproximal contact extending more apically, a broad zone of keratinized tissue, thick and fibrotic gingiva, and comparatively thick alveolar bone (Figure 4).8 In practical application, however, the diagnostic categories are often simplified to thick phenotype and thin phenotype.7
Another common term is gingival phenotype, which describes the phenotype of only the gingiva, not the underlying alveolar bone. Gingival phenotype comprises keratinized tissue width and gingival thickness. Because gingival phenotype can be measured in a noninvasive clinical fashion, it is commonly used in studies and by clinicians as a proxy for periodontal phenotype or to determine treatment needs.
Diagnosis and Related Factors
The diagnosis of periodontal phenotype is based on anatomic characteristics of the gingival phenotype (comprising gingival thickness and keratinized tissue width), bone morphotype, and tooth dimension.7 Ranges and average values for these anatomic features are listed in Table 1.
Each parameter of periodontal phenotype may be measured with different techniques. Keratinized tissue width can be assessed clinically with a periodontal probe or caliper measuring from the mucogingival junction to the free gingival margin.7,9 Gingival thickness is assessed noninvasively as a binary value by midfacial transmucosal probe visibility, ie, inserting a periodontal probe in the gingival sulcus and attempting to observe it through the soft tissues (Figure 5). Probe visibility is associated with gingival thickness of less than 1 mm, considered "thin," whereas lack of probe visibility is associated with gingival thickness of more than 1 mm, considered "thick." Gingival thickness can also be assessed numerically with transgingival probing (perpendicularly through the gingiva to bone, requiring anesthesia) or via imaging techniques such as cone-beam computed tomography (CBCT) or ultrasonography.9 Assessing bone morphotype requires procedures to visualize hard tissue such as surgery or CBCT.7
While exact criteria to diagnose periodontal phenotype are inconsistent in the literature, clinicians can use the mean dimensions enumerated in Table 1 and the descriptions of each diagnosis as provided by Zweers et al8 to guide their diagnosis. Prevalence of periodontal phenotype varies based on the assessment parameters used. When assessing gingival thickness, "thick" is observed slightly more than half of the time.7
Cairo and colleagues introduced the "recession type" (RT) diagnostic system, which has three categories. RT1 defects show no interproximal attachment loss; RT2 defects have mild interdental attachment loss that is less than or equal to the amount of facial attachment loss; and RT3 defects have greater interdental attachment loss than facial attachment loss.10 This system resolves some limitations of previous classifications,11 including the difficulty distinguishing between Miller class I and class II recessions, and incorporates both interdental bone and soft-tissue loss when categorizing recession type. Additionally, the RT system seeks to estimate the predictability of root coverage for each defect class.7 In treating RT1 defects, 100% root coverage can be expected; for RT2 defects, 100% root coverage may be possible depending on the procedure; and in RT3 defects, full root coverage is unachievable.10
The RT diagnostic system for gingival recessions also includes modifiers based on tooth conditions, specifically whether the cementoenamel junction (CEJ) is detectable and whether a step is present, indicating missing tooth structure.12 Class A is assigned if the CEJ is detectable; otherwise, Class B is designated. Sites without a step are considered negative (-), while those with a step are considered positive (+). Thus, a full diagnosis for a gingival recession may appear as "RT1-A-" in a site without interdental attachment loss with a detectable CEJ but without a step (Figure 1 and Figure 6). These criteria are summarized in Table 2, which presents a matrix that can be used to diagnose and develop a predictable treatment plan for each site based on gingival recession and phenotype parameters as well as tooth conditions.
Risk factors for gingival recession include thin periodontal or gingival phenotype, lack of or reduced (less than 2 mm) keratinized gingiva,13 history of periodontitis, history of gingival recession, bacterial inflammation, patient-mediated trauma (eg, aggressive or abrasive toothbrushing or oral piercings), or iatrogenic interventions (eg, subgingival crown margin, facial tooth movement, or surgical trauma).7,14
Periodontal Phenotype and Health
Several studies have examined the likelihood of long-term periodontal health and attachment level stability at sites without attached gingiva. All concluded that control of gingival inflammation via oral hygiene is paramount to maintenance of health, and periodontal health can be maintained regardless of phenotype. Only one study found that gingival inflammation persisted in areas of less than 2 mm keratinized and less than 1 mm attached gingiva despite effective oral hygiene,13 while others found that inflammation and health of the periodontium could be controlled despite lack of attached gingiva.15-18 It should be cautioned, however, that these studies evaluated patients with excellent oral hygiene, characterized by efficiency in plaque removal and delivery of minimal trauma to the periodontium. Further, some studies included extensive training in oral hygiene instruction prior to baseline measurements,16,17 and two of the studies were in dogs, for which oral hygiene was performed by researchers.15,18 One study also assessed disease progression in young, healthy dental students performing supervised oral hygiene following plaque disclosing.13 Such measures may not be generalizable to real-life scenarios for the provision of oral hygiene.
While an aspirational ideal, this level of oral hygiene may not be practically feasible for most patients in clinical practice, and capacity and motivation to perform high levels of hygiene may be fleeting in those who are capable. Thus, each treatment plan should be personalized based upon comprehensive oral evaluation and assessment of patient oral hygiene delivery and not on any singular finding.
Risks of Untreated Mucogingival Deformities
Due to differences in the mineral composition of the hard tissues of the tooth-namely enamel, cementum, and dentin-cementum and dentin are 10 times more susceptible to caries than is enamel.19,20 Thus, exposed root surfaces at sites with gingival recession are at an increased risk of developing radicular caries, and prevalence of radicular caries is high at sites with gingival recession. Because the prevalence, extent, and severity of gingival recession increase with age, the incidence of radicular caries in older age groups is often correlated with an underlying predisposition related to gingival recession.21 Radicular caries rates in patients aged 50 to 64 years old were approximately 26%,22 while in individuals aged 60 to 79 years old, the rates of radicular caries have been estimated to be between 65% to 95%.23,24 Further, the treatment prognosis of radicular caries is poor with 65% restoration survival rate at 2 years25 and equivocal results when standard topical fluoride applications are used to arrest radicular decay.26 In addition to the risks for dental caries, noncarious cervical lesions, caused by erosion, abrasion, and other factors, can also impact tooth structure exposed after gingival recession.27 These defects may threaten the structural integrity of teeth, cause dental sensitivity, lead to endodontic disease, or create unacceptable esthetics. Ultimately, prevention or correction of root exposure with gingival grafting and gingival phenotype modification may be the optimal treatment to prevent and/or arrest root pathologies.
In a long-term analysis of untreated buccal gingival recessions in patients with good oral hygiene over a mean follow-up period of 8.9 years, it was determined that these defects were highly likely to progress.5 In fact, 78% of defects had increased recession depth at follow-up.5 Further, sites demonstrating a lack and/or minimal width of keratinized tissue demonstrated greater clinical attachment loss than those with at least 2 mm of keratinized tissue present. The presence of keratinized tissue and greater keratinized tissue width-at baseline or after phenotype modification-reduces the likelihood of recession depth increase or new gingival recession development, according to this and other studies.5,28,29
Similarly, even in "ideal" patients (ie, systemically healthy, highly motivated, compliant patients with excellent oral hygiene) with mucogingival deformities, untreated sites demonstrated increases in mean gingival recession defect depth of approximately 30% over a 25-year period. Conversely, sites treated with free gingival grafts demonstrated reduction in gingival recession depth of more than 50% at 15 and 25 years post-treatment.4 Further, gingival recession at one site, while not always progressive, can indicate a risk development of recession defects at additional sites throughout the mouth. Patients with existing recession have a 2.16 times higher risk of developing new areas of recession.5 This suggests that individuals susceptible to gingival recession, particularly those with thin periodontal phenotypes, are likely to experience further recession without intervention.5
Although several theories explain how gingival recession could contribute to tooth loss (such as through progressive attachment loss or radicular caries), little evidence suggests that existing or worsening gingival recession leads to tooth loss.5
Periodontal Phenotype Modification Treatments
Periodontal phenotype modification therapy can involve the soft tissues (PhMT-s), hard tissues (PhMT-b), or both. Soft-tissue modification procedures seek to improve tissue quality; they aim to increase keratinized and attached tissue, enhance orofacial thickness, cover exposed root surfaces, increase vestibular depth, and/or address abnormal frenal attachments. Based on the previously discussed susceptibility of sites with thin periodontal phenotype to gingival recession, phenotype modification should be a primary treatment goal for any mucogingival procedure in the presence of a thin phenotype.
Various materials can be used for soft-tissue phenotype modification, including free gingival graft (FGG), connective tissue graft (CTG), acellular dermal matrix (ADM), collagen matrices, and a variety of biologics (Table 3). Flap techniques may include apically positioned flaps, coronally advanced flaps, replaced flaps, sulcular tunneling, and vestibular incision subperiosteal tunnel access (VISTA), and the procedure may involve vestibuloplasty or the release of muscular insertion or frena.
In deciding which technique to use for periodontal phenotype modification, goals of treatment must first be established. If root coverage is a goal of treatment, then the two best options for gingival phenotype modification are CTG and ADM, with CTG demonstrating the highest probability of achieving complete root coverage and most keratinized tissue gain as well as long-term maintenance of attained results.30,31 ADM provides an advantage in reduced morbidity because there is no need to harvest autogenous tissue. However, if root coverage is not a treatment goal, FGG becomes a compelling option due to it being the "gold standard for increasing [keratinized tissue]."30,32
Interventions prior to orthodontic therapy may be achieved with autogenous or allogeneic graft material to enhance gingival thickness and reduce the risk of gingival recession during orthodontic tooth movement. In patients with a pretreatment thin periodontal phenotype (as shown in Figure 1 and Figure 6), gingival grafting can result in significant changes in soft-tissue thickness (Figure 7).
Periodontal Phenotype and Restorative Treatment
Even in a clinically well-fitting dental restoration, a marginal gap of up to 200 µm may exist, which can result in biofilm accumulation.33,34 While supragingival margins are preferrable to promote periodontal health, esthetic demands or extensive caries may require restorative margins to be placed subgingivally. Subgingival restorations, however, are associated with plaque retention, inflammation, gingival recession, and a greater proportion of pathogenic, Gram-negative, anaerobic bacteria.35 Teeth with subgingival restorations and narrow zones of attached gingiva demonstrate greater inflammation and recession prevalence than those with subgingival restorations and wide zones of attached gingiva.35 In particular, 5 mm of keratinized gingiva and 3 mm of attached gingiva is necessary for long-term stability at sites with subgingival full-coverage restorations.36 When full-coverage restorations are planned, phenotype modification to increase the width of keratinized gingiva has been recommended at sites with less than 2 mm keratinized gingiva without optimal oral hygiene.35,37,38 Subgingival margin placement may lead to early gingival recession and attachment loss with an increased likelihood of recession seen at sites with a narrow band of attached gingiva.
If restorations are planned to be placed subgingivally, they should be designed to allow for a minimal marginal gap and high polish of circumgingival materials. Such factors will reduce, but not eliminate, plaque retentiveness and the risk of prosthesis-related periodontal inflammation.14 Phenotype modification in instances of altered passive eruption, short clinical crowns, and/or excessive gingival display may also be performed in cases with or without planned restorations for optimal esthetics and/or function (Figure 8 through Figure 10). In summary, ensuring there is adequate gingiva (at least 2 mm) around a tooth with an intracrevicular restoration will help maintain the longevity of the tooth and restoration, including its health, function, and esthetics.
Periodontal Phenotype and Orthodontics
Orthodontic tooth movement can be critical in achieving ideal smile esthetics and occlusion. Movement of teeth is dependent on the periodontium, and the underlying hard and soft tissues can be impacted by the force of tooth movement and the local environment. It has been estimated that 20% to 35% of patients may develop facial gingival recession within 5 years after orthodontic treatment.39 According to the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions, bony dehiscences after orthodontic tooth movement are more common in teeth surrounded by a thin periodontal phenotype or when the final position of teeth is outside the alveolar process.40 Furthermore, an increasingly large segment of orthodontic patients are adults,41,42 many of whom have thin periodontal phenotypes with less than 1 mm of facial bone.43 Even among younger patients, gingival sites with baseline keratinized tissue width of less than 2 mm demonstrated a greater incidence of lack of keratinized tissue (6.1%) than on teeth with more than 2 mm of keratinized tissue width (0.1%) after orthodontic tooth movement.44 Thin periodontal phenotype further increases the risk of complications such as bony dehiscence, gingival recession, and/or dental mobility during and after orthodontic treatment.
For these reasons, careful evaluation of the periodontal phenotype and 3-dimensional assessment of tooth movement and final tooth position relative to hard and soft tissues should be performed prior to the initiation of periodontal therapy.43 Fortunately, practices such as the use of CBCT and intraoral scanning of orthodontic patients can help clinicians better understand the periodontal phenotype and risks of orthodontic tooth movements before complications occur.45 Figure 11 through Figure 13 depict a sample case that demonstrates the need for phenotype modification prior to orthodontic treatment.
The concerns regarding orthodontic tooth movement in patients with thin periodontal phenotype are not new. Wennström in 1996 highlighted that tooth movement within the alveolar bony housing does not cause gingival recession.46 However, it was noted that thin gingiva resulting from facial tooth movement increases susceptibility to gingival recession after other local insults, including gingival inflammation and toothbrush trauma, leading to soft-tissue defects.46 Therefore, assessing buccolingual thickness of gingiva in the direction of tooth movement is crucial. Finally, the importance of meticulous plaque control throughout orthodontic therapy to maintain gingival health cannot be overstated.
Current evidence suggests that augmentation in the presence of thin phenotype or less than 2 mm keratinized tissue is recommended prior to labial tooth movement to reduce the risk of post-orthodontic complications.43 Current imaging and computer planning technologies allow for enhanced clinical predictions of potential orthodontic complications and can enable a more nuanced approach to identifying sites that may benefit from prophylactic or interventional phenotype modification. After the initial establishment of periodontal health, clinicians can consider prophylactic treatment of mucogingival deformities of teeth that will be moved outside the alveolar bone housing or otherwise repositioning the teeth within the alveolar bone housing and then providing any remaining necessary interventional treatment of mucogingival deformities.47
In addition to evaluation of the periodontal soft-tissue phenotype, hard-tissue phenotype modification (PhMT-b) allows for an increased scope of tooth movement, enabling expansion of the incisor relationship beyond Proffit's envelope by twofold.43 Additionally, such treatment can facilitate arch expansion, alleviate the need for premolar extraction, and improve tongue posture and airway opening.43 Furthermore, PhMT-b offers the opportunity to harness the regional acceleratory phenomenon to accelerate tooth movement and shorten treatment time for orthodontic tooth movement, often termed surgically facilitated orthodontic treatment (SFOT). Figure 14 and Figure 15 show a case requiring SFOT treatment due to expected lingual dehiscence; in this case, SFOT with PhMT-b allowed for arch expansion to the lingual and accelerated orthodontic treatment. SFOT with PhMT-b may also reduce orthodontic relapse after certain types of tooth movement.48 A lower chance of relapse is seen at teeth surrounded by thicker versus thinner cortical plates.49
Recommendations for Collaborative Care
Oral health professionals should recognize the importance of identifying and classifying periodontal phenotype and mucogingival deformities and their impact on oral health outcomes. Clinicians also need to understand the indications for intervention and phenotype modification. Because mucogingival deformities and related conditions around teeth are common,50,51 their identification can lead to improved treatment decisions and optimized restorative, orthodontic, and esthetic outcomes. Proper diagnosis of these conditions requires recording accurate and comprehensive periodontal measurements, including gingival margin location, width of keratinized and attached gingiva, and gingival thickness, at least annually.
Once periodontal phenotype and mucogingival deformities have been identified, practitioners should then assess underlying etiologies and formulate a treatment plan, including referral for phenotype modification. All treatment plans should include oral hygiene instructions, encompassing a thorough brushing technique with a soft-bristled toothbrush and appropriate pressure (recommendation of an electric toothbrush with a pressure sensor might be considered), and patient education on the consequences of these conditions. While not all mucogingival deformities require immediate referral, areas with evidence of progression and sites that are planned for subgingival restorations and orthodontic tooth movement should be evaluated and, if appropriate, treated expeditiously. Working together, periodontists and other oral health professionals can improve patients' oral health and quality of life through assessment and treatment of periodontal phenotype or mucogingival deformities.
Conclusion
Diagnosis of mucogingival deformities and thin periodontal phenotype can allow oral health professionals to improve the quality of treatments for patients. Identification and treatment of mucogingival deformities, including periodontal phenotype modification, can enable enhanced long-term maintenance of periodontal and oral health. Further, when considering restorative and/or orthodontic treatments, it is critical to assess the periodontal condition prior to initiating treatment to prevent breakdown. Soft-tissue quality and quantity are both critical components of a healthy periodontium for long-term maintenance.
Acknowledgment
Figure 11 through Figure 13 are courtesy of Jeff Vincent, DMD; Figure 14 and Figure 15 are courtesy of Deepali Rasila, BDS, MDS, and Kavita Nayar, BDS, MDS.
About the Authors
Ethan S. Madison, DDS
Periodontology Resident, University of Alabama at Birmingham School of Dentistry, Birmingham, Alabama
Hussein S. Basma, DDS, DESS, MS
Assistant Professor, Department of Periodontology, University of Alabama at Birmingham School of Dentistry, Birmingham, Alabama; Diplomate, American Board of Periodontology
Maria L. Geisinger, DDS, MS
Professor, Acting Chair, Program Director, Advanced Education in Periodontology, Department of Periodontology, University of Alabama at Birmingham School of Dentistry, Birmingham, Alabama; Diplomate, American Board of Periodontology
Queries to the author regarding this course may be submitted to authorqueries@conexiant.com.
References
1. Kim DM, Bassir SH, Nguyen TT. Effect of gingival phenotype on the maintenance of periodontal health: an American Academy of Periodontology best evidence review. J Periodontol. 2020;91(3):311-338.
2. Olsson M, Lindhe J. Periodontal characteristics in individuals with varying form of the upper central incisors. J Clin Periodontol.1991;18(1):78-82.
3. Bissada N, Boeriu S, eds. Clinical Periodontology & Implant Surgery: Translating Fundamental Questions into Clinical Practice. Kitchener, Ontario: Sorin Boeriu; 2020.
4. Agudio G, Chambrone L, Pini Prato G. Biologic remodeling of periodontal dimensions of areas treated with gingival augmentation procedure: a 25-year follow-up observation. J Periodontol.2017;88(7):634-642.
5. Chambrone L, Tatakis DN. Long-term outcomes of untreated buccal gingival recessions: a systematic review and meta-analysis. J Periodontol.2016;87(7):796-808.
6. Caton JG, Armitage G, Berglundh T, et al. A new classification scheme for periodontal and peri-implant diseases and conditions - introduction and key changes from the 1999 classification. J Periodontol.2018;89 suppl 1:S1-S8.
7. Cortellini P, Bissada NF. Mucogingival conditions in the natural dentition: narrative review, case definitions, and diagnostic considerations. J Periodontol. 2018;89 suppl 1:S204-S213.
8. Zweers J, Thomas RZ, Slot DE, et al. Characteristics of periodontal biotype, its dimensions, associations and prevalence: a systematic review. J Clin Periodontol.2014;41(10):958-971.
9. Pini Prato G, Di Gianfilippo R, Pannuti CM, et al. Diagnostic reproducibility of the 2018 Classification of Gingival Recession Defects and Gingival Phenotype: a multicenter inter- and intra-examiner agreement study. J Periodontol. 2023;94(5):661-672.
10. Cairo F, Nieri M, Cincinelli S, et al. The interproximal clinical attachment level to classify gingival recessions and predict root coverage outcomes: an explorative and reliability study. J Clin Periodontol.2011;38(7):661-666.
11. Miller PD Jr. A classification of marginal tissue recession. Int J Periodontics Restorative Dent. 1985;5(2):8-13.
12. Cairo F, Pini-Prato GP. A technique to identify and reconstruct the cementoenamel junction level using combined periodontal and restorative treatment of gingival recession. A prospective clinical study. Int J Periodontics Restorative Dent.2010;30(6):573-581.
13. Lang NP, Löe H. The relationship between the width of keratinized gingiva and gingival health. J Periodontol.1972;43(10):623-627.
14. Merijohn GK. Management and prevention of gingival recession. Periodontol 2000.2016;71(1):228-242.
15. Wennström J, Lindhe J. Role of attached gingiva for maintenance of periodontal health. J Clin Periodontol.1983;10(2):206-221.
16. Wennström JL. Lack of association between width of attached gingiva and development of soft tissue recession: a 5-year longitudinal study. J Clin Periodontol. 1987;14(3):181-184.
17. Kennedy JE, Bird WC, Palcanis KG, Dorfman HS. A longitudinal evaluation of varying widths of attached gingiva. J Clin Periodontol.1985;12(8):667-675.
18. Wennström J, Lindhe J. Plaque-induced gingival inflammation in the absence of attached gingiva in dogs. J Clin Periodontol.1983;10(3):266-276.
19. Hoppenbrouwers PM, Driessens FC, Borggreven JM. The mineral solubility of human tooth roots. Arch Oral Biol.1987;32(5):319-322.
20. Bowen WH. The Stephan Curve revisited. Odontology. 2013;101(1):2-8.
21. Heasman PA, Ritchie M, Asuni A, et al. Gingival recession and root caries in the ageing population: a critical evaluation of treatments. J Clin Periodontol. 2017;44 suppl 18:S178-S193.
22. Kirkegaard E, Borgnakke WS, Grønbæk L. Dental diseases, treatment needs and dental care habits in a representative segment of the adult Danish population [in Danish]. Tandlaegebladet. 1987;91(1):1-36.
23. Fure S, Zickert I. Prevalence of root surface caries in 55, 65, and 75-year-old Swedish individuals. Community Dent Oral Epidemiol.1990;18(2):100-105.
24. Salonen L, Allander L, Bratthall D, et al. Oral health status in an adult Swedish population. Prevalence of caries. A cross-sectional epidemiological study in the Northern Alvsborg county. Swed Dent J.1989;13(3):111-123.
25. Gil-Montoya JA, Mateos-Palacios R, Bravo M, et al. Atraumatic restorative treatment and Carisolv use for root caries in the elderly: 2-year follow-up randomized clinical trial. Clin Oral Investig. 2014;18(4):1089-1095.
26. Srinivasan M, Schimmel M, Riesen M, et al. High-fluoride toothpaste: a multicenter randomized controlled trial in adults. Community Dent Oral Epidemiol. 2014;42(4):333-340.
27. Teixeira DNR, Zeola LF, Machado AC, et al. Relationship between noncarious cervical lesions, cervical dentin hypersensitivity, gingival recession, and associated risk factors: a cross-sectional study. J Dent. 2018;76:93-97.
28. Kim DM, Neiva R. Periodontal soft tissue non-root coverage procedures: a systematic review from the AAP Regeneration Workshop. J Periodontol. 2015;86(2 suppl):S56-S72.
29. Chambrone L, Tatakis DN. Periodontal soft tissue root coverage procedures: a systematic review from the AAP Regeneration Workshop. J Periodontol. 2015;86(2 suppl):S8-S51.
30. Barootchi S, Tavelli L, Zucchelli G, et al. Gingival phenotype modification therapies on natural teeth: a network meta-analysis. J Periodontol. 2020;91(11):1386-1399.
31. Carbone AC, Joly JC, Botelho J, et al. Long-term stability of gingival margin and periodontal soft-tissue phenotype achieved after mucogingival therapy: a systematic review. J Clin Periodontol. 2024;51(2):177-195.
32. Zucchelli G, Tavelli L, McGuire MK, et al. Autogenous soft tissue grafting for periodontal and peri-implant plastic surgical reconstruction. J Periodontol. 2020;91(1):9-16.
33. Coli P, Karlsson S. Fit of a new pressure-sintered zirconium dioxide coping. Int J Prosthodont. 2004;17(1):59-64.
34. Chan C, Haraszthy G, Geis-Gerstorfer J, et al. Scanning electron microscopic studies of the marginal fit of three esthetic crowns. Quintessence Int. 1989;20(3):189-193.
35. Kosyfaki P, del Pilar Pinilla Martín M, Strub JR. Relationship between crowns and the periodontium: a literature update. Quintessence Int. 2010;41(2):109-126.
36. Maynard JG Jr, Wilson RD. Physiologic dimensions of the periodontium significant to the restorative dentist. J Periodontol.1979;50(4):170-174.
37. Stetler KJ, Bissada NF. Significance of the width of keratinized gingiva on the periodontal status of teeth with submarginal restorations. J Periodontol. 1987;58(10):696-700.
38. Nevins M. Attached gingiva-mucogingival therapy and restorative dentistry. Int J Periodontics Restorative Dent. 1986;6(4):9-27.
39. Renkema AM, Fudalej PS, Renkema A, et al. Development of labial gingival recessions in orthodontically treated patients. Am J Orthod Dentofacial Orthop. 2013;143(2):206-212.
40. Jepsen S, Caton JG, Albandar JM, et al. Periodontal manifestations of systemic diseases and developmental and acquired conditions: consensus report of workgroup 3 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol. 2018;89 suppl 1:S237-S248.
41. Saccomanno S, Saran S, Laganà D, et al. Motivation, perception, and behavior of the adult orthodontic patient: a survey analysis. Biomed Res Int. 2022;2022:2754051.
42. Hung M, Zakeri G, Su S, Mohajeri A. Profile of orthodontic use across demographics. Dent J (Basel). 2023;11(12):291.
43. Wang CW, Yu SH, Mandelaris GA, Wang HL. Is periodontal phenotype modification therapy beneficial for patients receiving orthodontic treatment? An American Academy of Periodontology best evidence review. J Periodontol. 2020;91(3):299-310.
44. Coatoam GW, Behrents RG, Bissada NF. The width of keratinized gingiva during orthodontic treatment: its significance and impact on periodontal status. J Periodontol. 1981;52(6):307-313.
45. Mandelaris GA, Neiva R, Chambrone L. Cone-beam computed tomography and interdisciplinary dentofacial therapy: an American Academy of Periodontology best evidence review focusing on risk assessment of the dentoalveolar bone changes influenced by tooth movement. J Periodontol. 2017;88(10):960-977.
46. Wennström JL. Mucogingival considerations in orthodontic treatment. Semin Orthod. 1996;2(1):46-54.
47. Chambrone L, Zadeh HH. Evidence-based rationale for the management of mucogingival deformities before or after orthodontic treatment. Semin Orthod. 2024;30(2):95-104.
48. Makki L, Ferguson DJ, Wilcko MT, et al. Mandibular irregularity index stability following alveolar corticotomy and grafting: a 10-year preliminary study. Angle Orthod. 2015;85(5):743-749.
49. Rothe LE, Bollen AM, Little RM, et al. Trabecular and cortical bone as risk factors for orthodontic relapse. Am J Orthod Dentofacial Orthop. 2006;130(4):476-484.
50. Romandini M, Soldini MC, Montero E, Sanz M. Epidemiology of mid-buccal gingival recessions in NHANES according to the 2018 World Workshop Classification System. J Clin Periodontol. 2020;47(10):1180-1190.
51. Strauss FJ, Marruganti C, Romandini M, et al. Epidemiology of mid-buccal gingival recessions according to the 2018 Classification System in South America: results from two population-based studies. J Clin Periodontol. 2023;50(10):1336-1347.