You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
Implant-supported restorations are a predictable therapeutic approach to replace missing teeth and restore oral health, function, and esthetics to both fully and partially edentulous patients.1 Long-term success of implant-supported restorations has been well documented in a variety of studies demonstrating implant success and survival rates above 95% in more than 10 years of follow-up.2-6
Despite the high success rates reported by dental clinicians, patients experience a variety of both implant and prosthetic problems.7 Biological complications include peri-implant mucositis, peri-implantitis, soft-tissue dehiscences, and inflammation under the prosthesis, while prosthetic issues may involve screw loosening and/or fracture as well as abrasion and/or veneer fracture of the restoration.7,8 While proper treatment planning and surgical execution at the time of implant placement can have a positive influence on many of these factors, peri-implant supportive therapy offers an opportunity to continuously monitor and assess existing complications and prevent future problems from occurring.
Proper peri-implant supportive therapy begins with correctly documenting any changes in the patient's medical and dental history, evaluating and providing instruction on the patient's home care, and performing complete periodontal charting supplemented with new radiographs if necessary.9-11 The clinical evaluation should also assess the soft-tissue thickness, amount of keratinization, and vestibular depth. Although the main stability of a dental implant depends on its osseointegration, the peri-implant soft tissue forms a barrier that is necessary for the longevity and maintenance of the implant.12
Anatomy and Physiology
Unlike natural dentition, dental implants lack root cementum and a periodontal ligament necessary for the insertion of Sharpey's fibers.13 Although a junctional epithelium hemidesmosomal attachment at the gingival cuff forms around implants, the adjacent gingival fibers of the connective tissue run parallel to the implant surface and do not insert into the actual dental implant.13 This results in a weaker gingival connection than is found in natural teeth where the fibers are perpendicular to the long axis of the tooth. Also, a longer biologic width is needed around implants as compared to natural dentition, and the connective tissue surrounding dental implants reportedly has a higher content of collagen and a lower content of fibroblasts, inflammatory cells, and blood vessels signifying a less rapid tissue turnover for peri-implant soft tissue.13 In addition, collagen fibers contain a higher content of collagen type V in healthy peri-implant tissues around one-piece dental implants.14
These differences in soft-tissue composition as well as the lack of a periodontal ligament reduce the total blood supply to the peri-implant tissues. Clinically, this presents as a more rapid breakdown of the supporting bone (compared to natural dentition) in the presence of inflammation due to contributing factors such as plaque and occlusal trauma.15 Therefore, meticulous peri-implant maintenance is critical especially because of the decreased ability for peri-implant tissues to arrest plaque-associated lesions.15 Maintenance of the peri-implant soft tissues may be as important to the long-term success of the implant as the initial osseointegration.16
Peri-implantitis
The formation and composition of subgingival bacterial plaque, also referred to as oral biofilm, on dental implants is similar to that of natural teeth, with only minor variations in plaque composition and bacterial adhesion rates.17 While initial plaque formation on dental implants for edentulous patients stems from the microflora of the patient's saliva, tonsils, and tongue, in partially edentulous patients opportunistic periodontal pathogens may be detected on dental implants in as little as 3 months after implant exposure.18,19 Additionally, within 6 months of implant placement, periodontal pathogens may be transmitted from any residual teeth to dental implants.20
Peri-implant mucositis, which is the precursor to peri-implantitis, develops after accumulation of bacterial biofilm on the implant surface, leading to an inflammatory reaction in adjacent soft tissue without loss of the supporting alveolar bone yet.21 Similar to periodontal disease, optimal plaque control and supportive therapy is vital to the prevention and subsequent maintenance of peri-implant disease. While peri-implant mucositis is reversible, if left untreated it may progress to peri-implantitis.
Peri-implantitis was defined at the 2017 World Workshop on the Classification of Periodontal and Peri-implant Diseases and Conditions as a plaque-associated pathologic condition occurring in the tissue around dental implants, characterized by inflammation in the peri-implant mucosa and subsequent loss of supporting bone.22 Peri-implantitis is the most common cause of implant failure after lack of osseointegration and is believed to be preceded by peri-implant mucositis.23,24 While dental implant placement has become a routine procedure to replace lost dentition, the prevalence of peri-implantitis persists,23,25 particularly as clinicians gain more understanding of the disease and are better able to identify it. A meta-analysis by Derks and Tomasi estimated the prevalence of peri-implant mucositis and peri-implantitis at 42.9% and 21.7%, respectively, including studies with a mean function time ranging from 3.4 to 11 years.26 Thus, with a proper maintenance protocol, peri-implant mucositis can be properly diagnosed and treated during routine maintenance visits while the inflammatory condition is still reversible.
Maintenance Evaluation
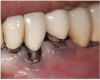
A degree of personalization is involved in implant maintenance therapy beginning with an evaluation of the patient's motivation and ability to maintain good oral hygiene prior to implant placement.27 Patient compliance is essential, and inadequate oral hygiene may be a contraindication to implant placement.10 Research shows that plaque buildup surrounding implants might exacerbate mucositis lesions after only 3 months of plaque accumulation.28 Therefore, similar to periodontal maintenance, peri-implant maintenance should be performed every 3 months.9,28 Clinical parameters for monitoring dental implants include probing depth, presence of bleeding on probing (BOP), suppuration, mucosal redness, plaque, keratinized mucosa, occlusion/prosthetic stability, and vestibular depth.10,11 Maintenance visits should also include radiographic evaluation if necessary, a review of the patient's medical history, and demonstration/evaluation of the patient's oral hygiene habits.9,10 Any changes in medical history, such as the onset of diabetes, and/or ability to maintain oral hygiene may affect the patient's susceptibility to peri-implantitis (Figure 1).
During maintenance visits, peri-implant probing depths should be measured with a force of 0.2 N, which is a lighter force than the probing around natural dentition because of the anatomical differences related to the gingival fiber orientation described above.29-31 While originally controversial, probing around dental implants does not cause irreversible damage to the implant or soft tissue, and the soft-tissue healing around the implant is expected to resolve after 5 to 7 days.32 It is also important to evaluate the quality of the soft tissue around dental implants during periodontal charting as this may be a predisposing factor to biological complications such as soft-tissue dehiscence and peri-implant mucositis.33,34
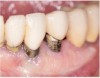
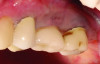
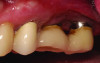
Tissue thickness has been shown to affect the initial crestal bone loss around dental implants.33 Linkevicius et al demonstrated that thick phenotypes had less initial crestal bone loss than thin phenotypes.33 However, when thin tissues were augmented with human soft tissues (allografts), the crestal bone loss was comparable to thick tissues.33 Therefore, a thicker phenotype is more desirable when evaluating soft-tissue quality around dental implants, and this aspect should be evaluated during maintenance visits. Additionally, the amount of keratinized tissue may affect the long-term prognosis of dental implants. In natural dentition, at least 2 mm of keratinized tissue reportedly is necessary to maintain health of the periodontal tissues.34,35 For dental implants, however, the significance of keratinized tissue in preserving implant health has been less clear, with some early studies showing no association between the amount of keratinized tissue and bone loss and some studies reporting that the presence of keratinized tissue significantly decreases the amount of recession and clinical attachment loss.34-36 Additional studies support a band of keratinized tissue of more than 2 mm to be associated with decreased BOP, plaque, mucosal recession, and attachment loss,34,37 especially when patients are not on a strict maintenance protocol.38 This may be because keratinized tissue provides increased comfort for the patient during home care regimens, whereas pain with brushing and therefore a reluctance to upkeep maintenance may occur with thin peri-implant mucosa (Figure 2).39 As a result, during supportive peri-implant therapy, adequate evaluation of soft-tissue quality around dental implants is crucial, with recommendations for soft-tissue augmentation if needed (Figure 3 and Figure 4).
Vestibular depth greatly affects patient comfort and the ability to access the implant during home care. Vestibular depth is measured from the mucosal margin to the point of greatest concavity of the mucobuccal fold during retraction.40 A study by Halperin-Sternfeld et al evaluated the relationship between vestibular depth and peri-implant parameters and noted a statistically significant increase in bone loss and recession around implants with a vestibular depth of less than 4 mm.40 Diagnosing a shallow vestibular depth during maintenance visits and correcting it with surgical intervention may be necessary to increase the long-term prognosis of the existing implant(s).
Soft-tissue quality can affect the susceptibility of dental implants to inflammation and breakdown. Additionally, assessing changes in the patient's medical history and any changes to their home care is important, further suggesting a degree of personalization to each patient's maintenance visit. Thorough clinical examinations, including checking occlusion and recording new radiographs, are necessary to avoid prosthetic complications such as screw loosening/fracturing and to enable earlier identification and treatment, if necessary, of peri-implant diseases.
Treatment of Peri-implant Diseases
Similar to the treatment of periodontitis and gingivitis, therapy for peri-implant diseases includes both nonsurgical and surgical methods.
Resective and Regenerative Surgical Approaches
Surgical treatment encompasses both resective and regenerative approaches.41 During resective surgical therapy of peri-implantitis, the objectives are to remove excess granulation tissue, halt osseous cratering, and apically position the tissues, all of which may be done in combination with implantoplasty to smooth the threads of the roughened implant surface (Figure 5 and Figure 6).41 Conversely, regenerative surgical therapy involves the use of various combinations of bone grafts, membranes, and biologic materials to regenerate the lost implant-supporting structures.42
Nonsurgical Therapy
Nonsurgical therapy is the first line of defense against peri-implant diseases in both treatment and prevention, with mechanical therapy the most utilized method for surface decontamination.43 Mechanical debridement can be used to remove plaque (biofilm) from the implant neck and abutment both supragingivally and subgingivally. For natural dentition, mechanical debridement is partly intended to remove diseased cementum; for dental implants, however, the implant surface is to be left unharmed. A variety of curettes may be utilized for the debridement of implant surfaces, each one with its advantages and disadvantages. Steel curettes have shown to alter the implant surface and cause damage because of their external hardness; therefore, careful use is recommended.44 Titanium curettes have a similar hardness to the implant surface and a comparable strength to steel curettes while causing less surface damage, although they may be less effective at removing calculus than steel curettes.45 Carbon-fiber curettes are softer than the implant surface and do not cause damage, but they are less effective at removing calculus than titanium and steel curettes and may break more easily.45 Teflon curettes have similar properties to carbon-fiber curettes and have been recommended as an adjunct to air-abrasive systems.45,46 Plastic curettes, the most fragile type of curettes, are ineffective at calculus removal at the implant surface and may leave plastic remnants in the peri-implant pocket that could further exacerbate the peri-implant disease.47
Curette selection may be case dependent based on the patient's existing peri-implant conditions and disease. When using curettes that have the potential to damage the implant surface, clinicians should be conscious of which type of stroke they are employing with the curette. Pull strokes with the potential to create vertical grooves on the implant surface may encourage epithelium downgrowth as opposed to horizontal grooves around the implant surface.48
Ultrasonic tips can also be used to debride the implant surface of existing calculus and biofilm. When debriding implants using ultrasonics, polyetheretherketone (PEEK)-coated tips are typically utilized; PEEK is a high-performance plastic material with a stainless steel core.45 Similar to curettes, however, the choice of ultrasonic tip may be case dependent.
Studies have shown that there is a greater cellular adherence to rough dental implant surfaces compared to smooth surfaces,49 a tendency known as "rugophilic." Implant surface roughness, therefore, plays a role in both soft-tissue adhesion and bacterial colonization on the implant surface,50 with decreased surface roughness lessening the accumulation of bacteria.51 Implant maintenance, thus, should include surface roughness evaluation.
As a substitute for mechanical debridement, air-polishing has been suggested as a way to remove biofilm from the implant without damaging its surface.41 Powders such as erythritol, sodium bicarbonate, and glycine have been utilized.52 While clinical studies have been unimpressive in terms of air-polishing resolving peri-implant mucositis when compared to the use of mechanical devices, in vitro research has shown it to have an impressive ability to remove biofilm from the implant surface.52 Air-polishing may be beneficial during routine periodontal maintenance appointments to aid in subgingival biofilm removal.
Dental Lasers
Dental lasers are useful in peri-implant therapy due to their ability to detoxify the implant surface without causing surface damage, assuming the right settings are utilized.53 Given the importance of laser wavelengths and parameters of laser-tissue interactions, the type of laser wavelength selected affects clinical efficacy.54 A laser can cause overheating of the implant and, therefore, leave irreversible changes to the implant surface and peri-implant tissues.55
Erbium-doped yttrium-aluminum-garnet (Er:YAG) lasers under appropriate settings, along with concomitant water spray to minimize temperature elevation, may be utilized directly on the implant surface without causing any surface alteration.56-58 An erbium, chromium: yttrium-scandium-gallium-garnet (Er,Cr:YSGG) laser has a similar absorption coefficient to the Er:YAG laser and, therefore, may also be used on a titanium implant surface with similar effects.56-58 An advantage of the Er:YAG laser is its absorption coefficient for hydroxyapatite and thus its ability to remove calculus.59 Schwarz and colleagues reported that in nonsurgical applications Er:YAG laser treatment led to substantial clinical improvements 6 months after therapy, with a significantly higher reduction of BOP compared to mechanical debridement with plastic instruments and antiseptic therapy.60,61
Carbon dioxide (CO2) lasers can be used to decontaminate an implant surface, with in vitro studies concluding that the CO2 laser does not damage the implant surface if proper power output and settings are utilized.62 Diode lasers have also shown to be beneficial as an adjunct to conventional debridement in the management of peri-implantitis, causing minimal changes in surface temperature.63,64 Additional in vivo research is necessary with longer-term follow-up and comparison of all wavelengths in the diode family.65
Laser treatment is beneficial when grafting is planned to cover exposed implant threads, as it allows for decontamination of the surface to be grafted.
Finally, photodynamic therapy (PDT) is an adjunctive method used to detoxify implant surfaces that has exhibited promising results in nonsurgical implant therapy. PDT involves the use of a photosensitizer that is activated by laser light to produce desired antimicrobial effects to decontaminate the implant surface. Having shown superior results in pockets less than 5 mm, PDT may be an excellent adjunct to mechanical therapy for supportive peri-implant care.66-68
As the knowledge base of dental laser therapy grows and is documented in the literature, ideal protocols with regard to laser type, power, and frequency settings (and photosensitizers when using PDT) will develop further, providing various approaches to treating implants with this modality.
Antimicrobials
Antimicrobials have been used as adjuncts to mechanical debridement when treating implants nonsurgically. Antimicrobials such as minocycline microspheres, tetracycline, and azithromycin have displayed promising results.69-71 Additionally, chlorhexidine (CHX) has been utilized as an adjunct to mechanical debridement to prevent recolonization of bacteria. However, a study by Heitz-Mayfield et al in which the test group used a chlorhexidine gel in addition to mechanical debridement failed to show any benefit of adding CHX as an adjunctive treatment modality.72 Moreover, CHX was shown to display persistent adsorption on the implant surface. Kotsakis et al demonstrated that residual CHX adversely affects osteoblasts and may compromise the biocompatibility of titanium surfaces,73 and an in vitro study by Kozlovsky et al showed a higher amount of adsorption on rough titanium disks when compared to smooth disks.74 CHX has also been shown to be cytotoxic toward fibroblasts and can potentially compromise soft-tissue healing.75 Additionally, combining CHX treatment with mechanical debridement using steel and titanium curettes may further roughen the implant surface, allowing for greater adsorption of CHX on the implant surface.
Since CHX use is not advised on implant surfaces,73 alternatives to it for antimicrobial treatment are sought-after. A novel herbal extract has emerged as a favored natural solution due to its selectively cytotoxic properties.76 While this oral care recovery product is often used in continuously expanding clinical applications, additional research is necessary to evaluate its substantivity on implant surfaces (both rough and smooth) and effects on supportive implant therapy.76
Conclusion
Supportive peri-implant care is critical to the success and longevity of dental implants. Peri-implant diseases, however, inevitably arise during maintenance and must be treated. Peri-implant mucositis, a plaque-induced, reversible inflammatory condition, can progress to peri-implantitis if left untreated, which underscores the importance of a regular maintenance protocol. The prevention of implant failure requires identification and treatment of peri-implant disease as early as possible to limit bone loss. Ongoing research is needed to refine treatment protocols and promote a thorough, individualized approach to peri-implant maintenance for long-term survival of dental implants.
Acknowledgment
Samuel L. Rabins served as study assistant. Dr. Estrin provided the cases shown in Figure 1 through Figure 6.
About the Authors
Nathan E. Estrin, DMD, MS
Adjunctive Faculty, College of Dentistry and Dental Clinics, University of Iowa, Iowa City, Iowa; Adjunctive Faculty, LECOM School of Dental Medicine, Bradenton, Florida; Private Practice, Sarasota, Florida; Diplomate, American Board of Periodontology
Jon B. Suzuki, DDS, PhD, MBA
Clinical Professor, University of Maryland, Baltimore, Maryland; Clinical Professor, University of Washington, Seattle, Washington; Clinical Professor, Nova Southeastern University, Fort Lauderdale, Florida; Professor Emeritus, Temple University, Philadelphia, Pennsylvania
Diana Bronstein, DDS, MS
Periodontist and Oral Implantologist, Master's of Science in Oral Biology, Medical Education, and Health Law; Diplomate, American Board of Periodontology; Diplomate and Fellow, International Congress of Oral Implantologists
Georgios Romanos, DDS, PhD, Prof Dr med dent
Professor, Department of Periodontology and Endodontics, School of Dental Medicine, Stony Brook University, Stony Brook, New York; Professor, Oral Surgeon, Johann Wolfgang Goethe University, Frankfurt, Germany; Diplomate, American Board of Periodontology
Queries to the author regarding this course may be submitted to authorqueries@conexiant.com.
References
1. Turkyilmaz I, Company AM, McGlumphy EA. Should edentulous patients be constrained to removable complete dentures? The use of dental implants to improve the quality of life for edentulous patients. Gerodontology. 2010;27(1):3-10.
2. Salinas TJ, Eckert SE. In patients requiring single-tooth replacement, what are the outcomes of implant- as compared to tooth-supported restorations? Int J Oral Maxillofac Implants. 2007;22 suppl:71-95.
3. Romanos GE, Gaertner K, Aydin E, Nentwig GH. Long-term results after immediate loading of platform-switched implants in smokers versus nonsmokers with full-arch restorations. Int J Oral Maxillofac Implants. 2013;28(3):841-845.
4. Romanos GE, Gaertner K, Nentwig GH. Long-term evaluation of immediately loaded implants in the edentulous mandible using fixed bridges and platform shifting. Clin Implant Dent Relat Res. 2014;16(4):601-608.
5. Buser D, Janner SF, Wittneben JG, et al. 10-year survival and success rates of 511 titanium implants with a sandblasted and acid-etched surface: a retrospective study in 303 partially edentulous patients. Clin Implant Dent Relat Res. 2012;14(6):839-851.
6. Fischer K, Stenberg T. Prospective 10-year cohort study based on a randomized controlled trial (RCT) on implant-supported full-arch maxillary prostheses. Part 1: sandblasted and acid-etched implants and mucosal tissue. Clin Implant Dent Relat Res. 2012;14(6):808-815.
7. Papaspyridakos P, Chen CJ, Singh M, et al. Success criteria in implant dentistry: a systematic review. J Dent Res.2012;91(3):242-248.
8. Estrin N, Nam K, Romanos GE, et al. Clinical outcomes of metal-ceramic vs metal-acrylic resin implant-supported fixed complete dental prostheses: a systematic review and meta-analysis. Int J Prosthodont. 2023;36(3):354-365.
9. Gulati M, Govila V, Anand V, Anand B. Implant maintenance: a clinical update. Int Sch Res Notices. 2014;2014:908534.
10. Humphrey S. Implant maintenance. Dent Clin North Am. 2006;50(3):463-478.
11. Lin CY, Chen Z, Pan WL, Wang HL. The effect of supportive care in preventing peri-implant diseases and implant loss: a systematic review and meta-analysis. Clin Oral Implants Res. 2019;30(8):714-724.
12. Lambrecht JT, Filippi A, Künzel AR, Schiel HJ. Long-term evaluation of submerged and nonsubmerged ITI solid-screw titanium implants: a 10-year life table analysis of 468 implants. Int J Oral Maxillofac Implants. 2003;18(6):826-834.
13. Thoma DS, Gil A, Hämmerle CH, Jung RE. Management and prevention of soft tissue complications in implant dentistry. Periodontol 2000. 2022;88(1):116-129.
14. Iezzi G, Di Lillo F, Furlani M, et al. The symmetric 3D organization of connective tissue around implant abutment: a key-issue to prevent bone resorption. Symmetry. 2021;13(7):1126.
15. Salvi GE, Aglietta M, Eick S, et al. Reversibility of experimental peri-implant mucositis compared with experimental gingivitis in humans. Clin Oral Implants Res. 2012;23(2):182-190.
16. Sclar AG. Soft Tissue and Esthetic Considerations in Implant Therapy. Batavia, IL: Quintessence Publishing; 2003.
17. Heitz-Mayfield LJ, Lang NP. Comparative biology of chronic and aggressive periodontitis vs. peri-implantitis. Periodontol 2000. 2010;53:167-181.
18. Mombelli A, Décaillet F. The characteristics of biofilms in peri-implant disease. J Clin Periodontol. 2011;38 suppl 11:203-213.
19. Teughels W, Van Assche N, Sliepen I, Quirynen M. Effect of material characteristics and/or surface topography on biofilm development. Clin Oral Implants Res. 2006;17 suppl 2:68-81.
20. Durrani F, Shukla A, Painuly H. Implant therapy in patients with chronic periodontitis: a short follow-up with a successful outcome. J Adv Periodontol Implant Dent. 2019;11(1):39-45.
21. Heitz-Mayfield LJA, Salvi GE. Peri-implant mucositis. J Periodontol. 2018;89 suppl 1:S257-S266.
22. Schwarz F, Derks J, Monje A, Wang HL. Peri-implantitis. J Periodontol.2018;89 suppl 1:S267-S290.
23. Hämmerle CH, Chen ST, Wilson TG Jr. Consensus statements and recommended clinical procedures regarding the placement of implants in extraction sockets. Int J Oral Maxillofac Implants. 2004;19 suppl:26-28.
24. Jepsen S, Berglundh T, Genco R, et al. Primary prevention of peri-implantitis: managing peri-implant mucositis. J Clin Periodontol. 2015;42 suppl 16:S152-S157.
25. Becker ST, Beck-Broichsitter BE, Rossmann CM, et al J. Long-term survival of Straumann dental implants with TPS surfaces: a retrospective study with a follow-up of 12 to 23 years. Clin Implant Dent Relat Res. 2016;18(3):480-488.
26. Derks J, Tomasi C. Peri-implant health and disease. A systematic review of current epidemiology. J Clin Periodontol. 2015;42 suppl 16:S158-S171.
27. Lang NP, Tonetti MS. Periodontal risk assessment (PRA) for patients in supportive periodontal therapy (SPT). Oral Health Prev Dent. 2003;1(1):7-16.
28. Zitzmann NU, Berglundh T, Marinello CP, Lindhe J. Experimental peri-implant mucositis in man. J Clin Periodontol. 2001;28(6):517-523.
29. Farina R, Filippi M, Brazzioli J, et al. Bleeding on probing around dental implants: a retrospective study of associated factors. J Clin Periodontol. 2017;44(1):115-122.
30. Bassir SH, Romanos GE. Significance of radiographic findings for the long-term success of dental implants. In: Romanos GE, ed. Saving Dental Implants. Wiley-Blackwell; 2024:18-26.
31. Herrera D, Berglundh T, Schwarz F, et al. Prevention and treatment of peri-implant diseases - the EFP S3 level clinical practice guideline. J Clin Periodontol. 2023;50 suppl 26:4-76.
32. Etter TH, Håkanson I, Lang NP, et al. Healing after standardized clinical probing of the perlimplant soft tissue seal: a histomorphometric study in dogs. Clin Oral Implants Res. 2002;13(6):571-580.
33. Linkevicius T, Puisys A, Linkeviciene L, et al. Crestal bone stability around implants with horizontally matching connection after soft tissue thickening: a prospective clinical trial. Clin Implant Dent Relat Res.2015;17(3):497-508.
34. Lin GH, Chan HL, Wang HL. The significance of keratinized mucosa on implant health: a systematic review. J Periodontol. 2013;84(12):1755-1767.
35. Wennström JL, Derks J. Is there a need for keratinized mucosa around implants to maintain health and tissue stability? Clin Oral Implants Res. 2012;23 suppl 6:136-146.
36. Tavelli L, Barootchi S, Avila-Ortiz G, et al. Peri-implant soft tissue phenotype modification and its impact on peri-implant health: a systematic review and network meta-analysis. J Periodontol.2021;92(1):21-44.
37. Gobbato L, Avila-Ortiz G, Sohrabi K, et al. The effect of keratinized mucosa width on peri-implant health: a systematic review. Int J Oral Maxillofac Implants. 2013;28(6):1536-1545.
38. Romanos G, Grizas E, Nentwig GH. Association of keratinized mucosa and periimplant soft tissue stability around implants with platform switching. Implant Dent. 2015;24(4):422-426.
39. Souza AB, Tormena M, Matarazzo F, Araújo MG. The influence of peri-implant keratinized mucosa on brushing discomfort and peri-implant tissue health. Clin Oral Implants Res. 2016;27(6):650-655.
40. Halperin-Sternfeld M, Zigdon-Giladi H, Machtei EE. The association between shallow vestibular depth and peri-implant parameters: a retrospective 6 years longitudinal study. J Clin Periodontol.2016;43(3):305-310.
41. Renvert S, Lindahl C, Roos Jansåker AM, Persson GR. Treatment of peri-implantitis using an Er:YAG laser or an air-abrasive device: a randomized clinical trial. J Clin Periodontol. 2011;38(1):65-73.
42. Renvert S, Polyzois I, Claffey N. Surgical therapy for the control of peri-implantitis. Clin Oral Implants Res. 2012;23 suppl 6:84-94.
43. Renvert S, Roos-Jansåker AM, Claffey N. Non-surgical treatment of peri-implant mucositis and peri-implantitis: a literature review. J Clin Periodontol. 2008;35(8 suppl):305-315.
44. Amodeo AA, Butera A, Lattari M, et al. Consensus report of the technical-scientific associations of Italian dental hygienists and the academy of advanced technologies in oral hygiene sciences on the non-surgical treatment of peri-implant disease. Int J Environ Res Public Health. 2023;20(3):2268.
45. Figuero E, Graziani F, Sanz I, et al. Management of peri-implant mucositis and peri-implantitis. Periodontol 2000. 2014;66(1):255-273.
46. Máximo MB, de Mendonça AC, Renata Santos V, et al. Short-term clinical and microbiological evaluations of peri-implant diseases before and after mechanical anti-infective therapies. Clin Oral Implants Res.2009;20(1):99-108.
47. Bertoldi C, Lusuardi D, Battarra F, et al. The maintenance of inserted titanium implants: in-vitro evaluation of exposed surfaces cleaned with three different instruments. Clin Oral Implants Res. 2017;28(1):57-63.
48. Rompen E, Domken O, Degidi M, et al. The effect of material characteristics, of surface topography and of implant components and connections on soft tissue integration: a literature review. Clin Oral Implants Res.2006;17 suppl 2:55-67.
49. bin Anwar Fadzil AF, Pramanik A, Basak AK, et al. Role of surface quality on biocompatibility of implants - a review. Ann 3D Printed Med.2022;8:100082.
50. Mehl C, Kern M, Schütte AM, et al. Adhesion of living cells to abutment materials, dentin, and adhesive luting cement with different surface qualities. Dent Mater. 2016;32(12):1524-1535.
51. Subramani K, Jung RE, Molenberg A, Hämmerle CH. Biofilm on dental implants: a review of the literature. Int J Oral Maxillofac Implants. 2009;24(4):616-626.
52. Atieh MA, Almatrooshi A, Shah M, et al. Airflow for initial nonsurgical treatment of peri-implantitis: a systematic review and meta-analysis. Clin Implant Dent Relat Res. 2022;24(2):196-210.
53. Aoki A, Mizutani K, Schwarz F, et al. Periodontal and peri-implant wound healing following laser therapy. Periodontol 2000. 2015;68(1):217-269.
54. Romanos G. Current concepts in the use of lasers in periodontal and implant dentistry. J Indian Soc Periodontol. 2015;19(5):490-494.
55. Romanos GE, Everts H, Nentwig GH. Effects of diode and Nd:YAG laser irradiation on titanium discs: a scanning electron microscope examination. J Periodontol. 2000;71(5):810-815.
56. Romanos G, Crespi R, Barone A, Covani U. Osteoblast attachment on titanium disks after laser irradiation. Int J Oral Maxillofac Implants. 2006;21(2):232-236.
57. Schwarz F, Nuesry E, Bieling K, et al. Influence of an erbium, chromium-doped yttrium, scandium, gallium, and garnet (Er,Cr:YSGG) laser on the reestablishment of the biocompatibility of contaminated titanium implant surfaces. J Periodontol. 2006;77(11):1820-1827.
58. Smeo K, Nasher R, Gutknecht N. Antibacterial effect of Er,Cr:YSGG laser in the treatment of peri-implantitis and their effect on implant surfaces: a literature review. Laser Dent Sci. 2018;2:63-71.
59. Estrin NE, Moraschini V, Zhang Y, et al. Combination of Nd: YAG and Er: YAG lasers in non-surgical periodontal therapy: a systematic review of randomized clinical studies. Lasers Med Sci. 2022;37(6):2737-2743.
60. Schwarz F, Sculean A, Rothamel D, et al. Clinical evaluation of an Er:YAG laser for nonsurgical treatment of peri-implantitis: a pilot study. Clin Oral Implants Res. 2005;16(1):44-52.
61. Schwarz F, Bieling K, Bonsmann M, et al. Nonsurgical treatment of moderate and advanced periimplantitis lesions: a controlled clinical study. Clin Oral Investig. 2006;10(4):279-288.
62. Romanos G, Ko HH, Froum S, Tarnow D. The use of CO(2) laser in the treatment of peri-implantitis. Photomed Laser Surg. 2009;27(3):381-386.
63. Arısan V, Karabuda ZC, Arıcı SV, et al. A randomized clinical trial of an adjunct diode laser application for the nonsurgical treatment of peri-implantitis. Photomed Laser Surg. 2015;33(11):547-554.
64. Lerario F, Roncati M, Gariffo A, et al. Non-surgical periodontal treatment of peri-implant diseases with the adjunctive use of diode laser: preliminary clinical study. Lasers Med Sci. 2016;31(1):1-6.
65. Estrin NE, Aoki A, Sculean A, et al. Lasers in surgical therapy of peri-implantitis. In: Romanos GE, ed. Saving Dental Implants. Wiley-Blackwell; 2024:266-286.
66. Vohra F, Al-Rifaiy MQ, Lillywhite G, et al. Efficacy of mechanical debridement with adjunct antimicrobial photodynamic therapy for the management of peri-implant diseases: a systematic review. Photochem Photobiol Sci. 2014;13(8):1160-1168.
67. Deppe H, Mücke T, Wagenpfeil S, et al. Nonsurgical antimicrobial photodynamic therapy in moderate vs severe peri-implant defects: a clinical pilot study. Quintessence Int. 2013;44(8):609-618.
68. Chambrone L, Wang HL, Romanos GE. Antimicrobial photodynamic therapy for the treatment of periodontitis and peri-implantitis: an American Academy of Periodontology best evidence review. J Periodontol. 2018;89(7):783-803.
69. Matesanz-Pérez P, García-Gargallo M, Figuero E, et al. A systematic review on the effects of local antimicrobials as adjuncts to subgingival debridement, compared with subgingival debridement alone, in the treatment of chronic periodontitis. J Clin Periodontol. 2013;40(3):227-241.
70. Schenk G, Flemmig TF, Betz T, et al. Controlled local delivery of tetracycline HCl in the treatment of periimplant mucosal hyperplasia and mucositis. A controlled case series. Clin Oral Implants Res. 1997;8(5):427-433.
71. Hallström H, Persson GR, Lindgren S, et al. Systemic antibiotics and debridement of peri-implant mucositis. A randomized clinical trial. J Clin Periodontol. 2012;39(6):574-581.
72. Heitz-Mayfield LJ, Salvi GE, Botticelli D, et al. Anti-infective treatment of peri-implant mucositis: a randomised controlled clinical trial. Clin Oral Implants Res. 2011;22(3):237-241.
73. Kotsakis GA, Lan C, Barbosa J, et al. Antimicrobial agents used in the treatment of peri-implantitis alter the physicochemistry and cytocompatibility of titanium surfaces. J Periodontol. 2016;87(7):809-819.
74. Kozlovsky A, Artzi Z, Moses O, et al. Interaction of chlorhexidine with smooth and rough types of titanium surfaces. J Periodontol. 2006;77(7):1194-1200.
75. Mariotti AJ, Rumpf DA. Chlorhexidine-induced changes to human gingival fibroblast collagen and non-collagen protein production. J Periodontol.1999;70(12):1443-1448.
76. Estrin NE, Romanos GE, Tatch W, et al. Biological characterization, properties, and clinical use of a novel homeopathic antiseptic oral recovery kit: a narrative review. Oral Health Prev Dent. 2022;20:485-499.