You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
Peri-implantitis is associated with local and systemic factors that significantly impact implant success.1 Successful peri-implantitis therapy is also multifactorial.2,3 Due to limited access to the implant surface, nonsurgical therapy alone has limited efficacy in treating most cases of peri-implantitis.4,5 Surgery, therefore, is often necessary for disease management.6
Peri-implant flap surgery is intended to enable access to the implant surface to remove biofilm soft and hard deposits and residual dental implant cement, thus promoting healing and reducing further progression. Reconstructive techniques seek to regenerate the bone defect and achieve reosseointegration7 while closing the pathogenic peri-implant pocket.8 The management of peri-implant defects relies mainly on understanding defect morphology and other related topographic characteristics.
Risk assessment tools and clinical checklists are beneficial to avoid undue complications.8-11 Several of the present authors have described a 10-key checklist for immediate implant placement at esthetic sites12,13 as well as a seven-key checklist intended for treating periodontal intrabony defects.14 All implant procedures involve the biological response of the host ("host response"), the microbiological environment ("microbiology"), and treatment factors associated with the human operator ("human factors").8,15 This article examines this process and presents eight keys for peri-implantitis defect regenerative treatment (Table 1). Each key can be linked to one or more of these three categories.
Eight Keys to Regenerative Treatment of Peri-Implantitis Defects
Key No. 1: Operator and Patient Factors, Implant Conditions, and Peri-implant Configuration (Human Factors)
The use of a checklist takes advantage of the collective wisdom of those who developed the checklist, providing consistent, effective, standardized treatment protocols.15 Interestingly, a growing focus across a range of industries, both in and outside of medicine and dentistry, has been on "human factors" as sources of error, including peri-implantitis treatments.9 Surgeon experience and skills and environmental stressors play critical roles in the success of these procedures.8 Techniques similar to those used in aviation industry checklists and situational awareness can help address human factor issues and improve clinical performance.1,10,16
When conducting Key No. 1, performing a full-mouth periodontal and occlusal assessment and radiographic evaluation (including full-mouth periapical and/or cone-beam computed tomography imaging) is recommended, along with an assessment of implant position and prosthesis, as these factors may contribute to peri-implantitis progression.6 In addition to the medical and dental history, the periodontal, occlusal, and radiographic examinations are assessed and reviewed with the patient in a pretreatment consultation "knee-to-knee and eye-to-eye." Based on previously published studies and treatment guidelines,17-20 this article introduces a peri-implantitis defect risk assessment (PIDRA) tool to aid clinicians in identifying influential factors to achieve successful outcomes when performing regenerative procedures (Table 2). The PIDRA allows for predictable, standardized patient and professional communication regardless of risk level.
Before commencing any peri-implant therapy, the potential for protheses adjustments or refabrication to optimize treatment outcomes must be considered. Therefore, patient-centered discussions regarding the possible need for this preparatory step and the timing and cost implications are essential before any nonsurgical or surgical intervention (Table 1).
Key No. 2: Nonsurgical Therapy (Human Factors)
The PIDRA assumes that the patient with a peri-implant defect is periodontally healthy and practices good dental hygiene. Nonsurgical (flapless) interventions are initially recommended to shift the biofilm ecosystems and assess their effectiveness. However, limited success has been reported for nonsurgical means in treating peri-implantitis. Hence, a re-evaluation after a period of at least 6 weeks post-nonsurgical treatment is essential to confirm disease resolution (ie, pocket depth less than 6 mm with no profuse bleeding on probing and no progressive bone loss). If these criteria are unmet, surgical therapy is advised based on the PIDRA risk profile (Table 2). A possible referral to a more experienced general dentist or specialist should be considered.
Key No. 3: Surgical Preparation (Host Response, Human Factors)
Three approaches have been proposed for peri-implantitis regeneration: (1) Submerged: remove implant crown, place cover screw, achieve primary closure over the grafted area, and allow for uninterrupted wound healing. (2) Non-submerged: keep the crown in situ and treat the defect similarly to natural teeth regeneration procedures. (3) Healing abutment: place a healing abutment to promote transmucosal healing; the prosthesis is then reinstalled following complete surgical site healing.
The submerged approach has three main shortcomings. The first is the inability to restore the implant immediately after surgery, especially in the esthetic zone, which may cause the flap to collapse. The second problem is that the crowns in these cases are not always retrievable, which means increased cost and time for fabricating a new crown. Thirdly, the coronally advancing flap used to attain primary closure will lead to coronally advancing the mucosal junction, a shallow vestibule, and insufficient buccal keratinized mucosa. This is especially a concern in molar sites.
While requiring additional steps and cost, removing the implants' suprastructures will provide increased visual access to the implant surface (ie, the valleys and threads of the implant) and the intrabony defect for detoxification and placement of regenerative materials. Posterior sites, especially molars, are more challenging to treat without direct visual access. This suggests that prosthesis removal with submerged healing may create a more favorable environment for bone regeneration.21,22
While the authors highly recommend antibiotic prophylaxis using 2 g of amoxicillin 1 hour before surgery, there is limited evidence on the effectiveness of any antibiotic protocol on the success of reconstructive therapy to manage peri-implantitis.
Key No. 4: Surgical Access (Host Response, Human Factors)
On the day of the surgical procedure, an Nd:YAG laser-assisted peri-implantitis procedure (LAPIP) may be employed to sterilize the submucosal diseased pocket while targeting the defect depth and adjacent inflamed soft tissues before raising a flap. This is followed by a marginal internal bevel incision using a papillary-sparing technique for access, which is done after completing full-thickness buccal and lingual flaps that extend half to one full tooth size in length on either side of the bony defect. The operator must confirm the type of defect visualized to determine the optimal surgical approach. Peri-implant defects may be classified according to their morphology and severity as follows23: Class I defects are intraosseous defects and subclassified as class Ia, buccal dehiscence; class Ib, two- to three-wall defect; and class Ic, circumferential defect. Class II defects are supracrestal/horizontal defects. Class III are combined defects, with subclassifications of class IIIa, buccal dehiscence + supracrestal bone loss; class IIIb, two- to three-wall defect + supracrestal bone loss; and class IIIc, circumferential defect + supracrestal bone loss. Diagnosing intraosseous defects is essential for guiding clinical treatment decisions.
Intrabony defects are best treated using regenerative methods, while peri-implant supracrestal defects are best addressed with resective therapy. Essentially, 25% of peri-implantitis defects have a combined (intrabony and supracrestal) defect configuration.23 Two studies have contributed to classifying peri-implant defects according to their morphology and severity (class I through class III).23,24 The most common defect configuration is Ib (two- to three-wall defect, with the buccal plate missing the bony wall). Defects Ia (buccal dehiscence), Ib, and IIIb (two- to three-wall defect + supracrestal bone loss) comprise roughly 86% of all defects.23
Class Ib cases may be associated with implants positioned beyond the confines of the osseous housing.25 In cases where primary wound closure may not be feasible, a combined therapeutic approach is recommended. This approach involves achieving pocket closure through resection techniques on the buccal aspect and utilizing reconstructive procedures at the interproximal aspect.
Soft-tissue augmentation using a connective tissue graft may be necessary for cases characterized by a thin periodontal phenotype (less than 2 mm of tissue thickness), an inadequate width of keratinized tissue, or areas of tissue shrinkage, as are commonly seen in the esthetic zone. Free epithelialized gingival grafts or soft-tissue alternatives can be used after at least 4 months post-reconstructive therapy in cases lacking an adequate zone (ie, less than 2 mm width) or thickness of attached keratinized gingiva. Tissue grafts have the added benefit of significantly reducing or eliminating gingival sensitivity that may be experienced during oral hygiene maintenance.
Key No. 5: Implant Surface Decontamination (Microbiology, Human Factors)
Removing the biofilm from the implant surface is essential for a successful outcome.26 Decontamination practices include the use of mechanical methods, such as titanium brushes, curettes, air-powder abrasive systems, ultrasonic tips, and implantoplasty; chemical agents, including hydrogen peroxide/citric acid, local tetracycline, 24% ethylenediaminetetraacetic acid (EDTA), and chlorhexidine; electrolytic cleaning; and laser energy.27,28 No method has been established as superior over the others.
A combined strategy may be the most effective approach.29 The authors favor the use of air-powder abrasive systems (either glycine or erythritol, with the particle size dependent on the specific product used) due to the ability to clean all aspects of the implant surface while causing the least amount of implant surface damage and release of titanium particles.
Key No. 6: Limitations of Reconstructive Therapy- Supracrestal Component and Areas Outside the Bony Housing (Host Response, Human Factors)
When treating a combined intrabony defect, the clinician's experience becomes increasingly crucial in selecting the most suitable and predictable treatment for the patient. The portion of the implant surface in the oral cavity extending from the peri-implant sulcus is at long-term risk if any roughened surface or threads are exposed. Such exposed roughened surface is significantly more susceptible to recontamination and recolonization than smooth implant surfaces. Therefore, performing implantoplasty must be considered to modify the roughened surface coronal to the intrabony defect, where some repair can be expected. When applicable, this should be completed before bone grafting using carbide football-shaped 12-fluted surgical-length burs under copious amounts of sterile water. The implantoplasty converts the roughened implant surface to a smooth-titanium tissue-level implant surface, making the local environment more amenable to soft-tissue reattachment and healing.30 Nevertheless, the impact of releasing titanium particles into the tissues is not well-established.
Key No. 7: Potential for Reconstruction (Human Factors)
"Reparative potential" refers to the intrabony component within the alveolar bony envelope.21 When more walls (three to four) are present, as seen with the treatment of intrabony defects around teeth, there is a significantly better prognosis for regeneration than a defect with fewer walls (one to two). In fact, a one-wall defect might not be indicated for reconstructive therapy.
Tissue regeneration relies on three key components: cells, scaffolds (eg, bone grafts), and signaling molecules (eg, growth factors). Vascularization, wound stability, and time are imperative for these components to successfully fulfill their role in tissue regeneration.31 Peri-implantitis defects involve significant bone loss and reduced blood and cellular supply. Based on the current evidence, the bone reconstruction of the defect should be performed using xenografts or allografts.
Because the stability of the wound is pivotal, barrier membranes may be used to support graft containment for partially contained defects (class Ib). In the case of a healing abutment approach (Key No. 3), trimming the membrane using the "poncho-like" technique and stabilizing it with the healing abutment has been evaluated with variable successes combined with allografts and xenografts.32 Conversely, in narrow circumferential defects (class Ia, IIIa), the use of a barrier membrane may not significantly enhance outcomes compared to not using a membrane.33-35
Unlike natural teeth, dental implants lack a surrounding periodontal ligament (PDL) necessary for regeneration; therefore, adding growth factors to the bone graft can compensate for the lack of PDL cells and promote more rapid bony healing of the defect. The recombinant human platelet-derived growth factor-BB (rhPDGF-BB) has been used with different bone substitutes, such as mineralized freeze-dried bone allograft (FDBA)36 or deproteinized bovine bone mineral (DBBM).37 Presently, the use of rhPDGF-BB in peri-implant regenerative procedures is considered an "off-label" means for treating peri-implantitis defects and is not available in many countries. Enamel matrix derivative (EMD) has been assessed for surgically treating peri-implantitis. Alberti et al proposed that EMD may improve bone and implant contact.38 Overall, only modest qualitative and quantitative evidence is available regarding the use of EMD to treat peri-implantitis.39
Finally, a critical factor in every successful regeneration procedure is the provision of tension-less primary closure over the surgical site. Periosteum-releasing incisions should be performed to achieve a tension-free closure of the flap. Recommended sutures are those that are nonresorbable, made of high-density polytetrafluoroethylene (d-PTFE), or are slowly resorbable, which should be left in for at least 4 weeks unless they become loose and, therefore, can be removed earlier.
Key No. 8: Supportive Care (Host Response, Microbiology, Human Factors)
The postoperative protocol can vary in complexity and approach across different practices. During postoperative visits the clinical team should have checklists for proper care of surgical patients, and the patient should understand that these visits are as critical to success as the procedure itself. Maintaining a plaque-free environment is crucial to minimizing the risk of re-infection post-surgery. Professional prophylaxis should be initiated in the first 2 to 3 weeks, with the use of a rubber cup or an air polisher device commencing after 4 to 6 weeks for plaque removal. Once the sutures are removed at 2 to 4 weeks, plaque control and polishing are recommended every 2 to 3 weeks for the first 3 months.40,41
Several considerations when treating peri-implantitis patients include: having a specific periodontal maintenance protocol (or supportive periodontal therapy [SPT]) and instrument setups, adequate appointed scheduled time for the SPT visit, and patient education, including using and sharing the patient's updated periodontal risk score (PRS) at each visit.10,16,42
Follow-up appointments should occur every 3 to 4 months. Use of an air polisher is recommended during the periodontal/peri-implant maintenance phase. Although there is limited evidence regarding the ideal frequency for peri-implant maintenance, scheduling visits every 3 months is advised, particularly during the first year.43 Later, maintenance visits may be customized according to the patient's risk profile.44 A recent study by Leone et al concluded that the likelihood of developing peri-implantitis is five times higher among noncompliant patients than among those who adhere to regular maintenance.45 Additionally, maintenance therapy at intervals of less than twice a year may be ineffective in preventing peri-implantitis.43,46
Case Report
The following case report describes treatment of a circumferential intrabony defect with a buccal dehiscence (class Ib-Ic defect), which was diagnosed clinically. In 2007, a 40-year-old nonsmoking woman (American Society of Anesthesiologists [ASA] 1) with excellent periodontal health visited a periodontist for the replacement of a single missing mandibular premolar (tooth No. 20), which had been extracted 6 months before. A restorative-driven tapered implant with an SLActive® surface (4.1 mm x 10 mm) was placed using a customized anatomically correct surgical guide. At 3 months, a 3-mm solid abutment was torqued to 35 Ncm, followed by a final porcelain-fused-to-metal crown cemented with resin cement. The postoperative care protocol was exclusively under the care of the restorative dentist post-completion, as the patient was periodontally healthy with an excellent PRS of 3.
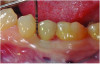
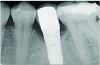
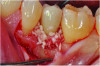
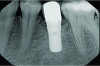
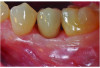
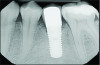
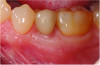
At 5 years post-implant placement, implant site No. 20 showed circumferential depths of 6 mm to 8 mm with heavy bleeding on probing (Figure 1).1,47 The keratinized mucosa width locally recorded 4 mm buccally and lingually, with a thick gingival phenotype. The four-wall intrabony lesion was diagnosed as a class 1c (circumferential) intrabony peri-implant defect with less than 40% defect angulation in the mesial and distal radiographic aspects (Figure 2).48 Additionally, resin cement-associated peri-implantitis was diagnosed.49 The patient preferred to maintain the existing well-fitting crown due to financial concerns. The patient would be considered low-medium risk according to the PIDRA (Table 3). (To view Table 3, the PIDRA for this case, visit compendiumce.com/go/2505.)
A minimally invasive papillary retention technique was used (to aid in flap closure), with buccal and lingual access flaps to gain visualization of the intrabony defect. The surgical goals were to remove all subgingival diseased biofilm and residual subgingival cement and perform guided bone regeneration of the defect.36,37,50 In this case, clinical expectation for the four-wall defect was to achieve bone fill to the level of the interproximal bone height of the adjacent teeth.14
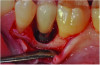
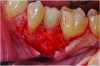
Curettes with small tips were used with ultrasonics to thoroughly clean the intrabony defect while not touching the implant surface (Figure 3 and Figure 4). The surface valleys and threads were carefully cleaned using an air polisher (sodium bicarbonate) for 1 minute, followed by sterile water irrigation of the site for 1 minute. After air drying, EDTA was applied for 1 minute, followed by sterile water rinse for 1 minute, with the previous steps repeated. The FDBA bone graft was soaked in sterile water, dried with a 2 x 2 gauze, and soaked in rhPDGF-BB (Figure 5).51 The bone graft was firmly packed into the defect with slight overpacking above the crest (Figure 6). A collagen membrane was divided in half and soaked in rhPDGF-BB. The collagen membranes were then adapted buccally and lingually with slight overlapping interproximally for graft containment (Figure 7). The flaps were passively positioned coronally and interproximally using nonresorbable 6-0 polypropylene sutures (Figure 8).
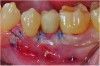
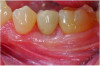
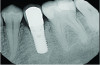
A postsurgical periapical x-ray was taken (Figure 9). Postoperative visits were at 2- to 3-week intervals. The patient was instructed to use chlorhexidine for 2 weeks, followed by the use of a two-row soft toothbrush and interproximal flossing. Interproximal proxy brush usage began after the suture removal at 4 weeks. After 2 months, healing was excellent (Figure 10); at 9 months, the interproximal papillae regeneration was complete, and radiographic confirmation of radiographic intrabony defect fill was observed (Figure 11 and Figure 12). After 1 year, maintenance visits were conducted by the restorative office's registered dental hygienist.
Six-year and 11-year recall visits (Figure 13 through Figure 15) confirmed stable long-term soft- and hard-tissue regeneration and bone healing.
Conclusion
Peri-implantitis-related lesions may be classified into human, host response, and microbiology-local factors. These factors are integrated into the peri-implant defect risk assessment (PIDRA) to gauge the potential level of difficulty, risk, and success associated with the treatment of an implant with peri-implantitis. Implementing the eight keys' checklist for treating peri-implantitis intrabony defects promotes a more standardized and predictable treatment outcome while reducing complications.
About the Authors
Robert A. Levine, DDS
Clinical Professor, Periodontology and Implantology, Kornberg School of Dentistry, Temple University, Philadelphia, Pennsylvania; Private Practice, Pennsylvania Center for Dental Implants and Periodontics, Philadelphia, Pennsylvania
Alberto Monje, DDS, MS, PhD
Adjunct Professor, Department of Periodontics and Oral Medicine, University of Michigan School of Dentistry, Ann Arbor, Michigan; Adjunct Professor, Department of Periodontology, School of Dentistry, Universitat Internacional de Catalunya, Barcelona, Spain
Muhammad H.A. Saleh, BDS, MSD, MS
Clinical Assistant Professor, Department of Periodontics and Oral Medicine, University of Michigan School of Dentistry, Ann Arbor, Michigan
Debora R. Dias, DDS, MSc
Assistant Professor, Department of Periodontics and Preventive Dentistry, University of Pittsburgh School of Dental Medicine, Pittsburgh, Pennsylvania; PhD Candidate, Department of Dentistry, State University of Maringá, Maringá, Brazil
Khushboo Kalani, DDS, MS
Periodontics Resident, Department of Periodontics and Oral Medicine, University of Michigan School of Dentistry, Ann Arbor, Michigan
Harold M. Pinsky, DDS
Private Practice, Ann Arbor, Michigan
Jeffrey Ganeles, DMD
Adjunct Associate Professor, Nova Southeastern University College of Dental Medicine, Fort Lauderdale, Florida; Private Practice in Periodontics and Implant Dentistry, Boca Raton, Florida
Franck Renouard, DDS
Private Practice, Paris, France
Maurício G. Araújo, DDS, MSc, PhD
Associate Professor, State University of Maringá, Maringá, Brazil; Private Practice in Dental Implants and Periodontics, Rio de Janeiro, Brazil
Queries to the author regarding this course may be submitted to authorqueries@conexiant.com.
References
1. Berglundh T, Armitage G, Araujo MG, et al. Peri-implant diseases and conditions: consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol. 2018;89 suppl 1:S313-S318.
2. Schwarz F, Sahm N, Schwarz K, Becker J. Impact of defect configuration on the clinical outcome following surgical regenerative therapy of peri-implantitis. J Clin Periodontol. 2010;37(5):449-455.
3. Schwarz F, Derks J, Monje A, Wang HL. Peri-implantitis. J Periodontol.2018;89 suppl 1:S267-S290.
4. Figuero E, Graziani F, Sanz I, et al. Management of peri-implant mucositis and peri-implantitis. Periodontol 2000. 2014;66(1):255-273.
5. Karlsson K, Derks J, Håkansson J, et al. Interventions for peri-implantitis and their effects on further bone loss: a retrospective analysis of a registry-based cohort. J Clin Periodontol.2019;46(8):872-879.
6. Saleh MHA, Galli M, Siqueira R, et al. The prosthetic-biologic connection and its influence on peri-implant health: an overview of the current evidence. Int J Oral Maxillofac Implants. 2022;37(4):690-699.
7. Renvert S, Polyzois IN. Clinical approaches to treat peri-implant mucositis and peri-implantitis. Periodontol 2000. 2015;68(1):369-404.
8. Renouard F, Renouard E, Rendon A, Pinsky HM. Increasing the margin of patient safety for periodontal and implant treatments: the role of human factors. Periodontol 2000. 2023;92(1):382-398.
9. Renouard F, Amalberti R, Renouard E. Are "human factors" the primary cause of complications in the field of implant dentistry? Int J Oral Maxillofac Implants. 2017;32(2):e55-e61.
10. Levine RA, Miller PD, Dias DR, et al. Translating clinical outcomes to patient value through use of the periodontal risk score: an evidence-based treatment approach. Compend Contin Educ Dent.2023;44(1):18-24.
11. Saleh MHA, Dias DR, Kumar P. The economic and societal impact of periodontal and peri-implant diseases. Periodontol 2000. 2024; doi: 10.1111/prd.12568.
12. Levine RA, Ganeles J, Gonzaga L, et al. 10 keys for successful esthetic-zone single immediate implants. Compend Contin Educ Dent. 2017;38(4):248-260.
13. Levine RA, Ganeles J, Kan J, Fava PL. 10 keys for successful esthetic-zone single implants: importance of biotype conversion for lasting success. Compend Contin Educ Dent. 2018;39(8):522-529.
14. Levine RA, Dias DR, Saleh MHA, et al. 7 keys for treatment of periodontal intrabony defects. Compend Contin Educ Dent. 2023;44(4):184-190.
15. Pinsky HM, Taichman RS, Sarment DP. Adaptation of airline crew resource management principles to dentistry. J Am Dent Assoc.2010;141(8):1010-1018.
16. Levine RA, Saleh MHA, Dias DR, et al. Periodontal regeneration risk assessment in the treatment of intrabony defects. Clin Adv Periodontics.2024;14(3):201-210.
17. Sinjab K, Garaicoa-Pazmino C, Wang HL. Decision making for management of periimplant diseases. Implant Dent.2018;27(3):276-281.
18. Monje A, Schwarz F. Principles of combined surgical therapy for the management of peri-implantitis. Clin Adv Periodontics.2022;12(1):57-63.
19. Schwarz F, Jepsen S, Obreja K, et al. Surgical therapy of peri-implantitis. Periodontol 2000. 2022;88(1):145-181.
20. Heitz-Mayfield LJA, Heitz F, Lang NP. Implant disease risk assessment IDRA - a tool for preventing peri-implant disease. Clin Oral Implants Res.2020;31(4):397-403.
21. Daugela P, Cicciu M, Saulacic N. Surgical regenerative treatments for peri-implantitis: meta-analysis of recent findings in a systematic literature review. J Oral Maxillofac Res.2016;7(3):e15.
22. Wen SC, Barootchi S, Huang WX, Wang HL. Surgical reconstructive treatment for infraosseous peri-implantitis defects with a submerged healing approach: a prospective controlled study. J Periodontol. 2022;93(2):195-207.
23. Monje A, Pons R, Insua A, et al. Morphology and severity of peri-implantitis bone defects. Clin Implant Dent Relat Res. 2019;21(4):635-643.
24. Schwarz F, Herten M, Sager M, et al. Comparison of naturally occurring and ligature-induced peri-implantitis bone defects in humans and dogs. Clin Oral Implants Res. 2007;18(2):161-170.
25. Rosen PS, Froum SJ, Sarmiento H, Wadhawani CP. A revised peri-implantitis classification scheme: adding three-dimensional considerations to facilitate prognosis and treatment planning. Int J Periodontics Restorative Dent. 2022;42(3):291-299.
26. Renvert S, Polyzois I, Maguire R. Re-osseointegration on previously contaminated surfaces: a systematic review. Clin Oral Implants Res.2009;20 suppl 4:216-227.
27. Roccuzzo M, Gaudioso L, Lungo M, Dalmasso P. Surgical therapy of single peri-implantitis intrabony defects, by means of deproteinized bovine bone mineral with 10% collagen. J Clin Periodontol.2016;43(3):311-318.
28. Roccuzzo M, Bonino F, Bonino L, Dalmasso P. Surgical therapy of peri-implantitis lesions by means of a bovine-derived xenograft: comparative results of a prospective study on two different implant surfaces. J Clin Periodontol. 2011;38(8):738-745.
29. Matarasso S, Quaremba G, Coraggio F, et al. Maintenance of implants: an in vitro study of titanium implant surface modifications subsequent to the application of different prophylaxis procedures. Clin Oral Implants Res. 1996;7(1):64-72.
30. Schwarz F, Hegewald A, John G, et al. Four-year follow-up of combined surgical therapy of advanced peri-implantitis evaluating two methods of surface decontamination. J Clin Periodontol.2013;40(10):962-967.
31. Langer R. Tissue engineering: perspectives, challenges, and future directions. Tissue Eng.2007;13(1):1-2.
32. Monje A, Pons R, Roccuzzo A, et al. Reconstructive therapy for the management of peri-implantitis via submerged guided bone regeneration: a prospective case series. Clin Implant Dent Relat Res. 2020;22(3):342-350.
33. Monje A, Pons R, Vilarrasa J, et al. Significance of barrier membrane on the reconstructive therapy of peri-implantitis: a randomized controlled trial. J Periodontol. 2023;94(3):323-335.
34. Regidor E, Ortiz-Vigón A, Romandini M, et al. The adjunctive effect of a resorbable membrane to a xenogeneic bone replacement graft in the reconstructive surgical therapy of peri-implantitis: a randomized clinical trial. J Clin Periodontol. 2023;50(6):765-783.
35. Roos-Jansåker AM, Persson GR, Lindahl C, Renvert S. Surgical treatment of peri-implantitis using a bone substitute with or without a resorbable membrane: a 5-year follow-up. J Clin Periodontol. 2014;41(11):1108-1114.
36. Froum SJ, Froum SH, Rosen PS. A regenerative approach to the successful treatment of peri-implantitis: a consecutive series of 170 implants in 100 patients with 2- to 10-year follow-up. Int J Periodontics Restorative Dent. 2015;35(6):857-863.
37. Froum SJ, Froum SH, Rosen PS. Successful management of peri-implantitis with a regenerative approach: a consecutive series of 51 treated implants with 3- to 7.5-year follow-up. Int J Periodontics Restorative Dent. 2012;32(1):11-20.
38. Alberti A, Francetti L, Taschieri S, Corbella S. The applications of enamel matrix derivative in implant dentistry: a narrative review. Materials (Basel). 2021;14(11):3045.
39. Moldovan R, Mester A, Piciu A, et al. Clinical outcomes of enamel matrix derivate used in surgical and non-surgical treatment of peri-implantitis: a systematic review of clinical studies. Medicina (Kaunas). 2022;58(12):1819.
40. Zucchelli G, Bernardi F, Montebugnoli L, De SM. Enamel matrix proteins and guided tissue regeneration with titanium-reinforced expanded polytetrafluoroethylene membranes in the treatment of infrabony defects: a comparative controlled clinical trial. J Periodontol. 2002;73(1):3-12.
41. Cortellini P, Bowers GM. Periodontal regeneration of intrabony defects: an evidence-based treatment approach. Int J Periodontics Restorative Dent.1995;15(2):128-145.
42. Saleh MHA, Tattan M, Troiano G, et al. Periodontal risk score: initiation and model validation for 6,762 teeth. J Periodontol.2023;94(4):459-466.
43. Monje A, Nart J. Disease recurrence during supportive therapy following peri-implantitis treatment: a retrospective study. J Periodontal Res.2024;59(5):918-928.
44. Monje A, Aranda L, Diaz KT, et al. Impact of maintenance therapy for the prevention of peri-implant diseases: a systematic review and meta-analysis. J Dent Res. 2016;95(4):372-379.
45. Leone FD, Blasi G, Amerio E, et al. Influence of the level of compliance with preventive maintenance therapy upon the prevalence of peri-implant diseases. J Periodontol.2024;95(1):40-49.
46. Monje A, Galindo-Fernández P, Nart J. Supportive therapy following peri-implantitis treatment: a retrospective study on compliance. Clin Oral Implants Res.2024;35(6):621-629.
47. Dukka H, Saleh MHA, Ravidà A, et al. Is bleeding on probing a reliable clinical indicator of peri-implant diseases? J Periodontol.2021;92(12):1669-1674.
48. Monje A, Pons R, Sculean A, et al. Defect angle as prognostic indicator in the reconstructive therapy of peri-implantitis. Clin Implant Dent Relat Res. 2023;25(6):992-999.
49. Wilson TG, Jr. Letter to the editor: Re: investigation of the association between cement retention and prevalent peri-implant diseases: a cross-sectional study. J Periodontol. 2016;87(9):998-999.
50. Wilson TG Jr, Valderrama P, Burbano M, et al. Foreign bodies associated with peri-implantitis human biopsies. J Periodontol.2015;86(1):9-15.
51. Jiang D, Dziak R, Lynch SE, Stephan EB. Modification of an osteoconductive anorganic bovine bone mineral matrix with growth factors. J Periodontol. 1999;70(8):834-839.