You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
Clinicians have numerous periodontal concepts available to them that can aid in the diagnosis and treatment of periodontal patients. The purpose of this article is to underscore contemporary viewpoints that should be considered when planning and administering nonsurgical periodontal treatment to individuals with localized or generalized periodontitis.
Etiology and Progression of Periodontal Diseases
Periodontal diseases are infections that can damage the periodontium.1 The term "infection" denotes a bacterial challenge and immune response.1 "Disease" indicates the presence of tissue destruction.2 Thus, periodontitis, which manifests an inflammatory infiltrate and bone and collagen degradation, is both an infection and a disease. Usually, the persistent intraoral bacterial challenge found supragingivally and subgingivally is controlled by the host response, and subclinical infections (ie, no visual signs of infection) do not typically cause disease.3 Accordingly, patients should be encouraged to maintain a high level of personal plaque control and comply with professional maintenance schedules to reduce the bacterial challenge, thereby facilitating stabilization of the host-parasite equilibrium.
Dahlen et al recently reviewed the etiology of periodontal diseases.4 Initially, they were thought to be instigated by bacteria in plaque (ie, biofilm); this was referred to as the nonspecific plaque hypothesis. Later, it was alleged that specific bacteria caused tissue destruction, and this was labeled the specific plaque hypothesis. Currently, periodontal diseases are believed to be caused by interactions between the polymicrobial community and a dysregulated inflammatory response, leading to dysbiosis and tissue destruction (Figure 1).5 The definitive role of specific bacteria in disease initiation and progression, however, remains unclear.4 At present, there is great interest regarding which risk factors (eg, genetics, smoking, systemic illnesses) predispose individuals to periodontal disease.6
More than 700 types of bacteria are found within the oral cavity,7 with the following five commonly associated with bacterial infections: Aggregatibacter (formerly Actinobacillus) actinomycetemcomitans, Porphyromonas gingivalis, Prevotella intermedia, Treponema denticola, and Tannerella forsythia.8 However, their mere presence is not a good predictor of future periodontal breakdown.9 In general, periodontal diseases are caused by mixed infections (ie, multiple pathogens), and patients who do not respond to scaling and root planing (SRP) may need adjunctive therapy, such as local or systemic antibiotic therapy, and/or periodontal surgery.10 In this regard, microbiological monitoring using culturing, DNA evaluations, or salivary diagnostics may be of benefit to guide therapy if patients are refractory to root instrumentation.
Bacteria associated with periodontal diseases are considered endogenous (part of the normal flora) versus exogenous (not normal members of the oral flora).11 Notably, it is difficult to eradicate endogenous bacteria, thus therapy needs to be directed at their suppression.12 Additionally, many bacteria have multiple clonal types (ie, genetic variations) that are not identified by culturing.13 For instance, there are 32 clonal types of P gingivalis, and some variants may be pathogenic and others benign.
Periodontal disease progression historically was thought to be slow and continuous.14 Subsequently, the episodic burst model suggested that disease progression occurred during short discontinuous intervals followed by periods of remission.14 This scheme, however, also has been questioned.15 Presently, it is recognized that there may be small microbursts of disease activity and that disease progression may be linear, or there may be larger episodes of tissue destruction lasting varying time periods followed by repair.14,15 Different patterns of disease development can occur at various sites within the same mouth or at a particular location at a different time. Also, it is recognized that gingivitis does not usually proceed to periodontitis, although gingivitis is frequently associated with periodontitis and often precedes it.16,17 Thus, the avoidance and elimination of gingivitis is prudent.
Diagnosis of Periodontitis
A number of key factors need to be considered in the diagnosis of periodontitis.
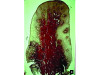
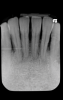
Bleeding: not a good predictor of disease activity. Bleeding on probing (BOP) represents an inflammatory infiltrate in the connective tissue, a reduction of collagen, and ulceration of the pocket's epithelial lining (Figure 2 and Figure 3).18 However, bleeding does not indicate the severity of periodontal lesions, and BOP is not a good predictor of disease activity.19 The term "disease activity" denotes there is ongoing clinical attachment and/or bone loss. Conversely, the absence of BOP is a good forecaster of clinical health and long-term tooth retention.19 Thus, BOP is an excellent indicator of the need for therapy, and its elimination is an important clinical endpoint.
Understanding biologic width, ie, supracrestal tissue attachment.20 Biologic width is the tissue coronal to the bone that attaches to the tooth. On average, there is usually 0.97 mm of junctional epithelium and 1.07 mm of connective tissue attachment,21 however these measurements can vary from 1 mm to 4 mm.22 Understanding the nuances of supracrestal tissue attachment evaluations can affect the interpretation of probing measurements and treatment decisions. For instance, in addressing the question of how deep a 6-mm probing depth actually is, intuitively the answer would be 6 mm. Histologically, however, this is not true. With a gentle probing pressure (25 gm)-a force that can blanch a nail bed-the probe penetrates almost through a healthy junctional epithelium (approximately 0.7 mm).23 In general, probing depths are around 1 mm greater than histologic probing depths, and a recorded 6-mm probing depth is actually a 5-mm histologic probing depth. The probing site is called a "pocket" if the tissues are inflamed; otherwise, in a healthy periodontium the measurement is referred to as probing depth. Also notable is that in a pocket with periodontitis the probe typically penetrates 0.3 mm into the connective tissue.24 Accordingly, a therapist must be aware of clinical probing depths when making treatment decisions for patients and when evaluating periodontal status after therapy.
Probing and pocket depths: not good predictors of disease activity. Teeth with deep pockets are considered at risk for further deterioration, as these sites, compared to those with shallow probing depths, manifest increased BOP and higher bacterial levels, are more difficult to achieve patient-performed plaque control to alter the subgingival microflora, reduce root instrumentation efficiency, and have an increased percentage of pockets with disease progression in untreated and periodontally treated sites.18,25 However, probing depth in untreated sites is a weak predictor of disease progression, because although deeper sites break down more often than shallow sites, the amount of clinical attachment or bone loss and the duration of time it takes to lose clinical attachment and bone are not highly predictable.18,25 Similarly, in treated sites, probing depth is a weak forecaster of future disease progression.18,25 For example, after nonsurgical therapy at healed sites, a 7-mm probing depth has a 50% chance of losing 1.5 mm of clinical attachment during the next 3.5 to 5 years.26 In contrast, the absence of deep probing depths is a good predictor of clinical health without disease progression.27
In summary, shallow probing depths are desirable but not always essential. The finding of a probing depth greater than 4 mm after therapy does not necessarily indicate an unfavorable prognosis for the tooth, but the patient should be periodically monitored.25 Practitioners performing probing assessments need to integrate the aforementioned information.
The use of dental radiographs to assess and forecast additional bone loss. Generally, radiographs underestimate the magnitude of bone loss by 9% to 20%.28 This is because the buccal and lingual bony plates hide the amount of osseous resorption. Thirty percent of crestal bone can be lost and several millimeters of clinical attachment loss can occur before bone loss is detected on a radiograph.29,30 A recent study indicated there is lack of complete clarity with respect to the relationship between bone and clinical attachment loss.31 In addition, it should be noted that the absence of crestal lamina dura is not a good indicator of disease progression.32 A radiograph is like a snapshot in time; it does not record ongoing disease progression. To validate that bone loss is occurring, clinical attachment measurements or radiographs need to be taken and monitored periodically. In this regard, computerized systems are being evaluated that can facilitate longitudinal assessment of alveolar bone changes, thereby providing objective criteria to monitor and manage periodontal patients.33
The degrees of bone loss detected on a radiograph in untreated and treated patients are not good predictors of future bone loss.18,25 This is particularly true in treated patients. Thus, bone loss needs to be interpreted in light of other clinical parameters to make the best judgment call with respect to ongoing disease activity. This is underscored by the updated periodontal classification scheme in which patients manifesting moderate signs of periodontitis are given a grade B designation.34 Grading of periodontitis reflects the rate of disease progression and is denoted as A (evidence of no bone loss over 5 years), B (<2 mm bone loss over 5 years), or C (>2 mm bone loss over 5 years).35 Without prior documentation, however, it is impossible to precisely determine a patient's rate of bone loss.
Mobility of teeth. Mobility can occur due to normal forces acting on a reduced periodontium (eg, a patient treated for periodontitis) or increased forces on a normal periodontium (eg, a patient with bruxism).18,36,37 Mobility results in resorption of bone and widening of the periodontal ligament. In a healthy periodontium, increased mobility does not cause clinical attachment loss or increased probing depths.37 Among periodontitis patients, mobility may or may not exacerbate disease progression.18,37,38 However, mobility is not necessarily progressive and cannot be used to predict tooth loss. Interestingly, hypermobility can cause a periapical area that looks like radiolucency associated with non-vital teeth (clinical observation). Thus, hypermobile teeth should not be judged to be non-vital based on radiographs. Therapy is needed for mobile teeth when there is increasing mobility, migration of teeth, pain on function, or if psychologically desired.25
Expectations After Scaling and Root Planing
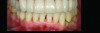
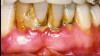
Numerous publications emphasize that oral hygiene and SRP to remove bacterial biofilms and calculus are essential facets of nonsurgical periodontal therapy.39-42 Subsequent to treatment, clinical signs of gingival inflammation (eg, redness), BOP, and probing depths are significantly reduced around single- and multi-rooted teeth (Figure 4 through Figure 9).41,42 A direct relationship has been reported between changes in clinical attachment level (CAL) and probing depth compared to initial pocket depth. The greatest gain of CAL and probing depth reduction occurs at locations with deep pockets (≥7 mm).40-42 The following clinical effects were noted after SRP: mean probing depth reductions at shallow pockets (1 mm to 3 mm), moderate sites (4 mm to 6 mm), and deep sites (>7 mm) were, respectively, 0.03 mm, 1.29 mm, and 2.16 mm.40 The average alterations in CAL for shallow, moderate, and deep probing sites were -0.34 mm (loss of CAL), 0.55 mm, and 1.19 mm, respectively (gain of CAL in the latter two groups).40 Root planing causes CAL loss at shallow probing sites (1 mm to 3 mm),40,42 because it removes cementum where Sharpey's fibers insert into the tooth.43 Proye et al showed recession may occur 1 week after root planing while a gain of CAL is seen in 3 weeks.44 These data indicate that patient alterations in probing depth and CAL may be reassessed 3 to 4 weeks after root instrumentation.
Waerhaug reported that a new junctional epithelium (approximately 1 mm long) develops within 2 weeks after mechanical therapy disrupts it.45 In this regard, Caton et al found that a long junctional epithelium (LJE) (average 2.5 mm) may develop at treated sites with advanced bone loss, and there is no new connective tissue attachment.46 From another standpoint, it was reported that locations with a LJE are not more disposed to disease progression than sites with a short junctional epithelium in the presence of good oral hygiene (monitored up to 6 months).47,48 Accordingly, after a maintenance visit, some probing sites may be deeper than originally measured due to disruption of the junctional epithelium. This may be particularly true at sites with previously treated infrabony defects that healed with a LJE.
Nonsurgical Mechanical Approaches
Hand versus power-driven instrumentation. Periodontal mechanical therapy can be accomplished with hand or power-driven devices (eg, sonic, ultrasonic).49-53 Benefits supplied by power-driven instruments are reduced fatigue, decreased working time, and the ability to access root furcations (approximately 1 mm wide) due to the thinness of ultrasonic tips.50,51 Nevertheless, these devices reduce tactile sensation.52,53 Systematic reviews have found no statistically significant differences regarding probing depth reduction, CAL gain, or decrease in microbial deposits when hand or power-driven instruments were compared for treatment of periodontitis.53-55 These findings support the idea that hand and ultrasonic instrumentation are both effective, and depending on the clinician's inclination, either manner of root cleansing is acceptable.
Full-mouth versus quadrant approach for root debridement.Historically, SRP was done one quadrant at a time. To increase efficiency, Quirynen et al suggested the notion of one-stage full-mouth disinfection employing full-mouth SRP.56 Theoretically, benefits of the full-mouth concept include the avoidance of cross-contamination from untreated pockets and fewer treatment visits.57 Critical analyses regarding this procedure did not find that full-mouth therapy provides a clinically relevant improvement beyond those results attained with partial-mouth therapy.58,59 On the other hand, another systematic review noted that for teeth with moderate probing depth (5 mm to 6 mm), full-mouth disinfection yielded minor benefits beyond single-quadrant-at-a-time SRP. The mean increase in probing depth reduction was 0.25 mm while CAL improvement was 0.33 mm.60 In summary, full-mouth and partial disinfection therapy are both effective treatment modalities for periodontitis, and the determining factor as to which one to use may be the clinician's and/or patient's preference.
Loss of tooth structure during nonsurgical mechanical therapy. Cementum is a hard, calcified layer of tissue that covers the root structure. It gets wider at the apex of a tooth and its thickness varies between 25 µm and 1,140 µm in maxillary molars and 20 µm to 700 µm in mandibular molars.61 The quantity of cementum removed during root planing is linked to several factors, including the modality of treatment (eg, sonic or ultrasonic scalers, hand instruments, or diamond burs), the softness of the diseased roots, pressure application, instrumentation time, the sharpness of the instrumentation, and the number of strokes used. Ritz et al reported the mean loss of cementum after root planing with 12 strokes using four different treatment methods. Cementum removal with ultrasonic scalers, sonic scalers, curettes, and diamond burs was, respectively, 11.6 µm, 93.5 µm, 108.9 µm, and 118.7 µm.62 These data validate that cementum is lost during root instrumentation. Thus, it is suggested that during therapy or maintenance visits, if there are no mechanically attached deposits on the roots and the periodontium is healthy, the sulci should only be deplaqued, as this avoids unnecessary cementum removal that could cause root sensitivity63 as well as loss of CAL in shallow probing depths.40
Other Considerations
Is cementum removal necessary to eliminate endotoxins? Endotoxin is a lipopolysaccharide found on roots of teeth and comes from cell walls of Gram-negative bacteria.64 Investigations noted the amount of endotoxin deposited on roots affected by periodontitis (20 ng to 394 ng) and on healthy roots (0.05 ng to 0.45 ng).65,66Historically, it was believed that cementum needed to be removed to eradicate endotoxin. It is presently understood that endotoxins are weakly bound to the root surface and can be eliminated with gentle washing, brushing, or ultrasonic instrumentation.67-71 Thus, aggressive cementum removal to provide a root surface free of endotoxin is unwarranted.
Regularity of maintenance visits. Periodontal maintenance is important for long-term success of periodontal patients.72,73Individuals with a history of periodontitis may need to be instrumented every 3 months to decrease their bacterial challenge so that their immune system can preserve homeostasis.72 The timeliness of maintenance visits needs be tailored to the patient's level of personal hygiene, periodontal history, and number of residual deep probing depths.73
Adjunctive therapies. Numerous adjunctive therapies can be used with SRP to try to enhance treatment results.74-82 Analysis of these data is beyond the scope of this article; however, Table 1 presents some interesting findings. It lists representative results achieved with root planing plus different therapeutic approaches (probiotics, lasers, photodynamic therapy, systemic antibiotics, and local drug delivery) from meta-analyses spanning 5 years.74-82 The results indicate there may be some modest benefits when adjunctive methods are used with root planing.
Table 2 illustrates the magnitude of probing depth reduction after multiple root planing visits.72,83-85 These results may vary depending on the severity of the initial problem, personal oral hygiene, time allocated for therapy, and skill of the therapist.
Notable Changes in Periodontal Classification
Finally, a number of key changes in the newest classification of periodontal diseases (2017) are worth noting86:
• Staging of periodontitis is characterized as I to IV based on severity of disease and complexity factors (eg, furcations).35
• Periodontitis is described based on its extent (localized or generalized). The terms "chronic" and "aggressive" periodontitis have been eliminated.
• Grading of periodontitis (rate of breakdown) is categorized as slow, moderate, and rapid progression and graded as A, B, and C, respectively.35
• Periodontal health is defined as absence of clinically detectable inflammation.
• The term "plaque induced" has been replaced by "dental biofilm induced."
• Risk factors added in the classification include smoking, hyperglycemia, nutritional factors, pharmacological agents, sex steroid hormones, and hematological condition.
• Non-plaque-induced gingival lesions are often manifestations of systemic conditions but may also represent pathologic changes limited to gingival tissues.
• The terms "acute necrotizing gingivitis" and "acute necrotizing periodontitis" have been replaced by "necrotizing gingivitis," "necrotizing periodontitis," and "necrotizing stomatitis," which are categorized under necrotizing periodontal diseases.
• Occlusal trauma has been renamed as traumatic occlusal force.
• The term "gingival biotype" has been replaced with "gingival phenotype."
Conclusions
Appropriate interpretation of clinical findings ensures that proper treatment protocols can be initiated in the treatment of periodontitis. This article reviewed many concepts whose application can enhance the diagnosis and treatment of periodontitis. SRP is a highly effective therapy for many patients. However, if the results of treatment are not satisfactory then adjunctive measures (eg, systemic antibiotics) and surgical debridement may be appropriate. Both nonsurgical and surgical treatments are effective modes of therapy, and clinicians need to decide which therapies are best for their patients based on the objectives (eg, pocket reduction, regeneration of bone, etc.) of therapy.
About the Authors
Gary Greenstein, DDS, MS
Former Clinical Professor, Department of Periodontology, College of Dental Medicine, Columbia University, New York, New York; Private Practice, Surgical Implantology and Periodontics, Freehold, New Jersey
Sultan Albeshri, BDS, MS
Assistant Professor, Department of Periodontics and Community Dentistry, College of Dentistry, King Saud University, Riyadh, Saudi Arabia
Queries to the author regarding this course may be submitted to authorqueries@conexiant.com.
References
1. Könönen E, Gursoy M, Gursoy UK. Periodontitis: a multifaceted disease of tooth-supporting tissues. J Clin Med. 2019;8(8):1135.
2. van Seventer JM, Hochberg NS. Principles of infectious diseases: transmission, diagnosis, prevention, and control. Int Encyclopedia Pub Health. 2017:22-39. doi: 10.1016/B978-0-12-803678-5.00516-6.
3. Silva N, Abusleme L, Bravo D, et al. Host response mechanisms in periodontal diseases. J Appl Oral Sci. 2015;23(3):329-355.
4. Dahlen G, Fejerskov O, Manji F. Current concepts and an alternative perspective on periodontal disease. BMC Oral Health. 2020;26:20(1):235.
5. Kornman KS, Page RC, Tonetti MS. The host response to the microbial challenge in periodontitis: assembling the players. Periodontol 2000. 1997;14:33-53.
6. Abou El Fadl RK, Abdel Fattah MA, Helmi MA, et al. Periodontal diseases and potential risk factors in Egyptian adult population - results from a national cross-sectional study. PLoS One. 2021;16(11):e0258958.
7. Deo PN, Deshmukh R. Oral microbiome: unveiling the fundamentals. J Oral Maxillofac Pathol. 2019;23(1):122-128.
8. Hyvärinen K, Laitinen S, Paju S, et al. Detection and quantification of five major periodontal pathogens by single copy gene-based real-time PCR. Innate Immun. 2009;15(4):195-204.
9. Chew RJJ, Goh CE, Sriram G, et al. Microbial biomarkers as a predictor of periodontal treatment response: a systematic review. J Periodontal Res. 2023;58(6):1113-1127.
10. Barca E, Cifcibasi E, Cintan S. Adjunctive use of antibiotics in periodontal therapy. J Istanb Univ Fac Dent. 2015;49(3):55-62.
11. Ruby J, Barbeau J. The buccale puzzle: the symbiotic nature of endogenous infections of the oral cavity. Can J Infect Dis. 2002;13(1):34-41.
12. Bertolini M, Costa RC, Barão VAR, et al. Oral microorganisms and biofilms: new insights to defeat the main etiologic factor of oral diseases. Microorganisms. 2022;10(12):2413.
13. Ali RW, Martin L, Haffajee AD, Socransky SS. Detection of identical ribotypes of Porphyromonas gingivalis in patients residing in the United States, Sudan, Romania and Norway. Oral Microbiol Immunol. 1997;12(2):106-111.
14. Reddy MS, Geurs NC, Jeffcoat RL, et al. Periodontal disease progression. J Periodontol. 2000;71(10):1583-1590.
15. Nagarajan R, Miller CS, Dawson D III, Ebersole JL. Biologic modelling of periodontal disease progression. J Clin Periodontol. 2019;46(2):160-169.
16. Murakami S, Mealey BL, Mariotti A, Chapple ILC. Dental plaque-induced gingival conditions. J Periodontol. 2018;89 suppl 1:S17-S27.
17. Sheiham A. Is the chemical prevention of gingivitis necessary to prevent severe periodontitis? Periodontol 2000. 1997;15:15-24.
18. Lang NP, Bartold PM. Periodontal health. J Periodontol. 2018;89 suppl 1:S9-S16.
19. Lang NP, Adler R, Joss A, Nyman S. Absence of bleeding on probing. An indicator of periodontal stability. J Clin Periodontol. 1990;17(10):714-721.
20. Jepsen S, Caton JG, Albandar JM, et al. Periodontal manifestations of systemic diseases and developmental and acquired conditions: Consensus report of workgroup 3 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol. 2018;89 supp 1:S237-S248.
21. Gargiulo AW, Wentz FM, Orban B. Dimensions and relations of the dentogingival junction in humans. J Periodontol. 1961;32(3):261-267.
22. Vacek JS, Gher ME, Assad DA, et al. The dimensions of the human dentogingival junction. Int J Periodontics Restorative Dent. 1994;14(2):154-165.
23. Caton J, Greenstein G, Polson A. Depth of periodontal probe penetration related to clinical and histologic signs of gingival inflammation. J Periodontol. 1981;52(10):626-629.
24. Armitage GC. Periodontal diseases: diagnosis. Ann Periodontol. 1996;1(1):37-215.
25. Greenstein G, Greenstein B, Cavallaro J. Prerequisite for treatment planning implant dentistry: periodontal prognostication of compromised teeth. Compend Contin Educ Dent. 2007;28(8):436-446.
26. Claffey N, Nylund K, Kiger R, et al. Diagnostic predictability of scores of plaque, bleeding, suppuration and probing depths for probing attachment loss. 3½ years of observation following initial periodontal therapy. J Clin Periodontol. 1990;17(2):108-114.
27. Armitage GC, Svanberg GK, Löe H. Microscopic evaluation of clinical measurements of connective tissue attachment levels. J Clin Periodontol. 1977;4(3):173-190.
28. Akesson L, Hakansson J, Rohlin M. Comparison of panoramic and intraoral radiography and pocket probing for the measurement of the marginal bone level. J Clin Periodontol. 1992;19(5):326-332.
29. Ortman LF, McHenry K, Hausmann E. Relationship between alveolar bone measured by 125I absorptiometry with analysis of subtraction radiographs: 2. Bjorn technique. J Periodontol. 1982;53(5):311-314.
30. Goodson JM, Haffajee AD, Socransky SS. The relationship between attachment level loss and alveolar bone loss. J Clin Periodontol. 1984;11(5):348-359.
31. Farook FF, Alodwene H, Alharbi R, et al. Reliability assessment between clinical attachment loss and alveolar bone level in dental radiographs. Clin Exp Dent Res. 2020;6(6):596-601.
32. Greenstein G, Polson A, Iker H, Meitner S. Associations between crestal lamina dura and periodontal status. J Periodontol. 1981;52(7):362-366.
33. Zaki HA, Hoffmann KR, Hausmann E, Scannapieco FA. Is radiologic assessment of alveolar crest height useful to monitor periodontal disease activity? Dent Clin North Am. 2015;59(4):859-872.
34. Kornman KS, Papapanou PN. Clinical application of the new classification of periodontal diseases: ground rules, clarifications and "gray zones." J Periodontol. 2020;91(3):352-360.
35. Papapanou PN, Sanz M, Buduneli N, et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol. 2018;89 suppl 1:S173-S182.
36. Ghods K, Alaee A, Jafari A, Rahimi A. Common etiologies of generalized tooth mobility: a review of literature. J Res Dent Maxillofac Sci. 2022;7(4):249-259.
37. Fan J, Caton JG. Occlusal trauma and excessive occlusal forces: narrative review, case definitions, and diagnostic considerations. J Clin Periodontol. 2018;45 suppl 20:S199-S206.
38. Harrel SK, Nunn ME, Hallmon WW. Is there an association between occlusion and periodontal destruction?: Yes-occlusal forces can contribute to periodontal destruction. J Am Dent Assoc. 2006;137(10):1380-1384.
39. Smiley CJ, Tracy SL, Abt E, et al. Systematic review and meta-analysis on the nonsurgical treatment of chronic periodontitis by means of scaling and root planing with or without adjuncts. J Am Dent Assoc. 2015;146(7):508-524.e5.
40. Cobb CM. Non-surgical pocket therapy: mechanical. Ann Periodontol. 1996;1(1):443-490.
41. Meseli SE, Kuru B, Kuru L. Relationships between initial probing depth and changes in the clinical parameters following non-surgical periodontal treatment in chronic periodontitis. J Istanb Univ Fac Dent. 2017;51(3):11-17.
42. Cobb CM, Sottosanti JS. A re-evaluation of scaling and root planing. J Periodontol. 2021;92(10):1370-1378.
43. Schüpbach P, Gaberthüel T, Lutz F, Guggenheim B. Periodontal repair or regeneration: structures of different types of new attachment. J Periodontal Res. 1993;28(4):281-293.
44. Proye M, Caton J, Polson A. Initial healing of periodontal pockets after a single episode of root planing monitored by controlled probing forces. J Periodontol. 1982;53(5):296-301.
45. Waerhaug J. Healing of the dento-epithelial junction following subgingival plaque control. II: As observed on extracted teeth. J Periodontol. 1978;49(3):119-134.
46. Caton JG, Zander HA. The attachment between tooth and gingival tissues after periodic root planing and soft tissue curettage. J Periodontol. 1979;50(9):462-466.
47. Beaumont RH, O'Leary TJ, Kafrawy AH. Relative resistance of long junctional epithelial adhesions and connective tissue attachments to plaque-induced inflammation. J Periodontol. 1984;55(4):213-223.
48. Magnusson I, Runstad L, Nyman S, Lindhe J. A long junctional epithelium-a locus minoris resistentiae in plaque infection? J Clin Periodontol. 1983;10(3):333-340.
49. Walmsley AD, Lea SC, Landini G, Moses AJ. Advances in power driven pocket/root instrumentation. J Clin Periodontol. 2008;35(8 suppl):22-28.
50. Barendregt DS, van der Velden U, Timmerman MF, van der Weijden F. Penetration depths with an ultrasonic mini insert compared with a conventional curette in patients with periodontitis and in periodontal maintenance. J Clin Periodontol. 2008;35(1):31-36.
51. Mengel R, Stelzel M, Mengel C, et al. An in vitro study of various instruments for root planing. Int J Periodontics Restorative Dent. 1997;17(6):592-599.
52. Oosterwaal PJ, Matee MI, Mikx FH, et al. The effect of subgingival debridement with hand and ultrasonic instruments on the subgingival microflora. J Clin Periodontol. 1987;14(9):528-533.
53. Krishna R, De Stefano JA. Ultrasonic vs. hand instrumentation in periodontal therapy: clinical outcomes. Periodontol 2000. 2016;71(1):113-127.
54. Muniz FWMG, Langa GPJ, Pimentel RP, et al. Comparison between hand and sonic/ultrasonic instruments for periodontal treatment: systematic review with meta-analysis. J Int Acad Periodontol. 2020;22(4):187-204.
55. Suvan J, Leira Y, Moreno Sancho FM, et al. Subgingival instrumentation for treatment of periodontitis. A systematic review. J Clin Periodontol. 2020;47 suppl 22:155-175.
56. Quirynen M, Bollen CM, Vandekerckhove BN, et al. Full- vs. partial-mouth disinfection in the treatment of periodontal infections: short-term clinical and microbiological observations. J Dent Res. 1995;74(8):1459-1467.
57. Quirynen M, De Soete M, Dierickx K, van Steenberghe D. The intra-oral translocation of periodontopathogens jeopardises the outcome of periodontal therapy. A review of the literature. J Cin Periodontol. 2001;28(6):499-507.
58. Santuchi CC, Cortelli JR, Cortelli SC, et al. Scaling and root planing per quadrant versus one-stage full-mouth disinfection: assessment of the impact of chronic periodontitis treatment on quality of life-a clinical randomized, controlled trial. J Periodontol. 2016;87(2):114-123.
59. Eberhard J, Jervøe-Storm PM, Needleman I, et al. Full-mouth treatment concepts for chronic periodontitis: a systematic review. J Clin Periodontol. 2008;35(7):591-604.
60. Fang H, Han M, Li QL, et al. Comparison of full-mouth disinfection and quadrant-wise scaling in the treatment of adult chronic periodontitis: a systematic review and meta-analysis. J Periodontal Res. 2016;51(4):417-430.
61. Stamfelj I, Vidmar G, Cvetko E, Gaspersic D. Cementum thickness in multirooted human molars: a histometric study by light microscopy. Ann Anat. 2008;190(2):129-139.
62. Ritz L, Hefti AF, Rateitschak KH. An in vitro investigation on the loss of root substance in scaling with various instruments. J Clin Periodontol. 1991;18(9):643-647.
63. Draenert ME, Jakob M, Kunzelmann KH, Hickel R. The prevalence of tooth hypersensitivity following periodontal therapy with special reference to root scaling. A systematic review of the literature. Am J Dent. 2013;26(1):21-27.
64. Raetz CR, Whitfield C. Lipopolysaccharide endotoxins. Annu Rev Biochem. 2002;71:635-700.
65. Maidwell-Smith M, Wilson M, Kieser JB. Lipopolysaccharide (endotoxin) from individual periodontally involved teeth. J Clin Periodontol. 1987;14(8):453-456.
66. Jones WA, O'Leary TJ. The effectiveness of in vivo root planing in removing bacterial endotoxin from the roots of periodontally involved teeth. J Periodontol. 1978;49(7):337-342.
67. Moore J, Wilson M, Kieser JB. The distribution of bacterial lipopolysaccharide (endotoxin) in relation to periodontally involved root surfaces. J Clin Periodontol. 1986;13(8):748-751.
68. Smart GJ, Wilson M, Davies EH, Kieser JB. The assessment of ultrasonic root surface debridement by determination of residual endotoxin levels. J Clin Periodontol. 1990;17(3):174-178.
69. Nyman S, Westfelt E, Sarhed G, Karring T. Role of "diseased" root cementum in healing following treatment of periodontal disease. A clinical study. J Clin Periodontol. 1988;15(7):464-468.
70. Hughes FJ, Smales FC. Immunohistochemical investigation of the presence and distribution of cementum-associated lipopolysaccharides in periodontal disease. J Periodontal Res. 1986;21(6):660-667.
71. Blomlöf L, Lindskog S, Appelgren R, et al. New attachment in monkeys with experimental periodontitis with and without removal of the cementum. J Clin Periodontol. 1987;14(3):136-143.
72. Magnusson I, Lindhe J, Yoneyama T, Liljenberg B. Recolonization of a subgingival microbiota following scaling in deep pockets. J Clin Periodontol. 1984;11(3):193-207.
73. Cohen RE; Research, Science and Therapy Committee, American Academy of Periodontology. Position paper: periodontal maintenance. J Periodontol. 2003;74(9):1395-1401.
74. Ho SN, Acharya A, Sidharthan S, et al. A systematic review and meta-analysis of clinical, immunological, and microbiological shift in periodontitis after nonsurgical periodontal therapy with adjunctive use of probiotics. J Evid Based Dent Pract. 2020;20(1):101397.
75. Li MM, Jia JH, Wu MX, et al. Clinical effectiveness of Er,Cr:YSGG lasers in non-surgical treatment of chronic periodontitis: a meta-analysis of randomized controlled trials. Lasers Med Sci. 2021;36(4):889-901.
76. Lin Z, Strauss FJ, Lang NP, et al. Efficacy of laser monotherapy or non-surgical mechanical instrumentation in the management of untreated periodontitis patients. A systematic review and meta-analysis. Clin Oral Investig. 2021;25(2):375-391.
77. Xue D, Tang L, Bai Y, et al. Clinical efficacy of photodynamic therapy adjunctive to scaling and root planing in the treatment of chronic periodontitis: a systematic review and meta-analysis. Photodiagnosis Photodyn Ther. 2017;18:119-127.
78. Salvi GE, Stähli A, Schmidt JC, et al. Adjunctive laser or antimicrobial photodynamic therapy to non-surgical mechanical instrumentation in patients with untreated periodontitis: a systematic review and meta-analysis. J Clin Periodontol. 2020;47 suppl 22:176-198.
79. Zhao H, Hu J, Zhao L. Adjunctive subgingival application of chlorhexidine gel in nonsurgical periodontal treatment for chronic periodontitis: a systematic review and meta-analysis. BMC Oral Health. 2020;20(1):34.
80. Herrera D, Matesanz P, Martín C, et al. Adjunctive effect of locally delivered antimicrobials in periodontitis therapy: a systematic review and meta-analysis. J Clin Periodontol. 2020;47 suppl 22:239-256.
81. Khattri S, Kumbargere Nagraj S, Arora A, et al. Adjunctive systemic antimicrobials for the non-surgical treatment of periodontitis. Cochrane Database Syst Rev. 2020;11(11):CD012568.
82. Albeshri S, Greenstein G. Efficacy of nonsurgical periodontal therapy for treatment of periodontitis: practical application of current knowledge. Gen Dent. 2022;70(5):12-19.
83. Torfason T, Kiger R, Selvig KA, Egelberg J. Clinical improvement of gingival conditions following ultrasonic versus hand instrumentation of periodontal pockets. J Clin Periodontol. 1979;6(3):165-176.
84. Listgarten MA, Lindhe J, Hellden L. Effect of tetracycline and/or scaling on human periodontal disease. Clinical, microbiological, and histological observations. J Clin Periodontol. 1978;5(4):246-271.
85. Badersten A, Nilveus R, Egelberg J. Effect of nonsurgical periodontal therapy. III. single versus repeated instrumentation. J Clin Periodontol. 1984;11(2):114-124.
86. Rajesh KS, Kumar A, Reshmi TS, et al. An overview on new classification of periodontal and peri-implant diseases and conditions 2017. J Dent Med Sci. 2020;19(7):52-55.
![Fig 1. Pathogenesis of periodontitis. The host response to a bacterial challenge can be both protective and destructive and results in a cascade of events, which culminates in connective tissue and alveolar bone loss. Initially, neutrophils, monocytes, and macrophages respond to the bacterial challenge. If they cannot control the pathogens, these cells release inflammatory mediators called cytokines (eg, interleukin-1 [IL-1], tumor necrosis factor-alpha [TNF-α]), which prompt other cells (normal epithelial and fibroblasts) to release prostaglandins (eg, PGE2) and matrix metalloproteinases (MMPs) (eg, collagenase). PGE2 can cause bone dissolution, and MMPs induce collagen degradation and some bone resorption.](/media/thumbnail/33865)