You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
The popularity of zirconia as a dental restorative material has increased dramatically in recent years. Having a high flexural strength and resistance to fracture, zirconia has physical properties that make this material highly advantageous for use as a dental restorative. High-strength zirconia has a flexural strength and resistance to fracture higher than that of both lithium disilicate and feldspathic porcelain.1-4 Zirconia has excellent wear resistance, with minimal wear to opposing tooth structure when properly polished.5-7 Zirconia also offers flexibility to the dental practitioner, as it can be bonded or conventionally cemented depending on clinician preferences and the clinical requirements of the case, and it is CAD/CAM compat-
ible. In addition, with the introduction of "translucent" zirconia, the esthetics of monolithic zirconia have improved greatly in recent years. Zirconia can be cut back or milled as a supporting framework and pressed or layered with various porcelains to further optimize esthetics. These and many other advantages have contributed to the increasing use of zirconia in restorative dentistry.
FORMS OF ZIRCONIA
Zirconia has three different and distinct crystalline configurations-monoclinic, tetragonal, and cubic-each of which is temperature- and pressure-dependent and has different physical and optical properties.8 At room temperature, zirconia exists in the monoclinic crystalline form, which is very stable but does not have the physical and optical properties that would enable its use as a dental restorative. However, when heated to approximately
1,170°C, the monoclinic powdered form of zirconia coalesces into
a solid (sintering), and the zirconia crystals undergo a phase transformation to the tetragonal crystalline configuration. Transformation to the cubic crystalline configuration (commonly known as cubic zirconia) occurs when the zirconia is heated to approximately 2,100°C.9 The cubic crystalline configuration of zirconia produces a very hard, translucent, and somewhat brittle material popularly known as cubic zirconia. While it is primarily the tetragonal form of zirconia that is used in the fabrication of dental restorations, cubic crystals, in varying amounts, can be incorporated into the zirconia matrix to improve translucency. The tetragonal configuration of zirconia is very strong, biocompatible, and corrosion resistant, can be milled using CAD/CAM technology, and is a suitable material for dental restorations and supporting substructures. However, because the tetragonal form of zirconia is not inherently stable, it easily converts back to the more stable but weaker monoclinic crystalline form. To stabilize the tetragonal crystalline form, dopants (a small amount of an impurity element introduced into a material to alter its electrical, physical, and optical properties) such as yttrium oxide (Y2O3) and aluminum oxide (Al2O3) are added.10
However, this "yttria-stabilized zirconia" is not entirely stable. Rather, it is what is referred to as "metastable," because under certain conditions the tetragonal crystals can convert back into their monoclinic configuration.10,11 This property of metastability has advantages with regard to the durability of the zirconia, as it contributes to a phenomenon known "transformation toughening," which promotes resistance to crack propagation; briefly explained, with the initiation of a crack, a localized conversion of the tetragonal crystals into the monoclinic form occurs, causing a volumetric expansion of the crystals around the emerging crack, sealing the crack and inhibiting its propagation. While the metastable nature of zirconia can be favorable because of transformation toughening, excessive tetragonal to monoclinic phase transformation can weaken overall assembly strength, with potentially disastrous consequences.12-14 It is therefore important for dentists to realize that "all zirconia is not created equal," and that because even minor errors in processing temperatures can result in short-term or long-term failure,12-14 only zirconia from reputable manufacturers, laboratories, and dealers should be used.
LAYERED VS MONOLITHIC ZIRCONIA RESTORATIONS
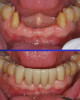
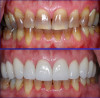
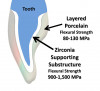
Zirconia restorations may be either monolithic (solid zirconia throughout, Figure 1) or layered (having a porcelain overlay, Figure 2). To obtain the greatest benefit from the physical properties of zirconia, zirconia should be used as full contour monolithic restorations whenever feasible.1-3 When zirconia is layered with ceramics (often to optimize esthetics) the layering ceramic, as well as the interface between the zirconia and the layered cera-
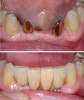
mic, are weak links in the restorative assembly (Figure 3 and Figure 4).
The esthetics of monolithic zirconia have been improved greatly in recent years with the introduction of the so-called "translucent" zirconia, manufactured through a variety of techniques, including manipulating dopant levels (typically by increasing the yttria concentration) to increase the proportion of the cubic crystals relative to the more opaque tetragonal crystals.15
It should be noted that, as regards translucent zirconia restorations, esthetics and strength are essentially inversely correlated; as the translucency increases, flexural strength and crack resistance decrease. This is because the more translucent the zirconia is, the more cubic crystals (which provide translucency) there are relative to tetragonal crystals. It is the tetragonal crystals that
undergo transformation toughening, so when fewer tetragonal crystals are present, the zirconia is less crack resistant.
SANDBLASTING ZIRCONIA PRIOR TO PLACEMENT
Sandblasting (airborne particle abrasion) is often employed to optimize adhesion between dental restorative materials and both conventional and resin-based cements. Sandblasting optimizes the restoration substrate by cleaning the surface of impurities, increasing surface roughness and surface area, raising surface energy, and improving the bond to subsequently placed primers, adhesives, and both conventional and resin-based cements.16It is the author's opinion that, when zirconia is used, the intaglio zirconia surface should always be sandblasted prior to placement of the restoration, regardless of the type of conventional or resin-based cement that is used. This recommendation has substantial support in the literature.15,17-20 Although concern has been raised that this surface treatment may induce surface and subsurface damage,21,22 to the best of the author's knowledge there have been no in vivo studies that show this to be a clinical problem, provided that proper blasting pressures, particle types, and particle sizes are used. With regard to sandblasting protocols, researchers generally concur that traditional high-strength zirconia (3 mol% to 4 mol% yttria concentration) can be safely and effectively airborne-particle-abraded with 30 to 50 micron aluminous oxide (Al2O3) using a blast pressure of 1.5 to 2.8 bar (approximately 20 to 40 psi) at a distance of 1 to 2 cm for 10 to 20 seconds (Dr. Nasser Barghi, Dr. Byoung Suh, personal communication, 2015).20,23 It has also been recommended that the nozzle head of the sandblaster be positioned at an angle (not perpendicular) relative to the zirconia surface (N. Barghi, B. Suh, personal communication, 2015). In the case of translucent zirconia (5 mol% yttria concentration), because this material is less capable of transformation toughening than traditional high-strength zirconia, surface damage that could reduce the physical properties of the zirconia should be minimized through the use of lower blasting pressures (approximately 20 psi).24 In the author's experience, the intaglio surface of zirconia restorations should be sandblasted after try-in and any adjustments, and just before placement of the restoration with conventional cementing or adhesive bonding.
USE OF ZIRCONIA PRIMERS AND CLEANING SOLUTIONS
To optimize the bond between zirconia and resin-based cements, a zirconia primer should be used after sandblasting.25 A separately applied solution that contains a phosphate ester zirconia primer such as a 10-MDP (10-methacryloyloxydecyl dihydrogen phosphate) primer can be used (eg, Z-Prime™ Plus, BISCO, Inc.; Monobond Plus, Ivoclar; Clearfil Ceramic Primer Plus, Kuraray; various universal adhesives18) or a resin cement that incorporates a zirconia primer (eg, PANAVIA™ SA Cement Plus, Kuraray Noritake Dental; 3M™ RelyX™ Unicem 2, 3M ESPE; SpeedCEM Plus, Ivoclar; TheraCem®, BISCO, Inc.). Phosphate ester primers such as 10-MDP chemically interact with the zirconia surface by both hydrogen and ionic bonding mechanisms.26 Zirconia has a strong affinity for phosphate ions,27 which are present in both zirconia primers and saliva. Thus, when zirconia restorations are tried in and the intaglio surface is contaminated by saliva, the phosphate ions from the saliva bind to and occupy the same reactive sites required for the chemical interactions with the primers. As the phosphate ions of the saliva compete with those of the zirconia primer, the effectiveness of the primer is decreased. To "free-up" these sites so that the zirconia primer can function optimally, the restoration should therefore be sandblasted and/or a strongly alkaline cleaning solution (ie, Ivoclean [Ivoclar] or ZirClean® [BISCO, Inc.]) used after the saliva contamination has occurred.28,29 (It is important to note that vigor-
ous rinsing with water and/or the use of acetone and alcohol and phosphoric acid are not effective in cleaning saliva-contaminated zirconia surfaces.30) Because strongly alkaline cleaning solutions have a higher affinity for phosphate ions than does the zirconia, they are able to chemically remove phosphate ions from the surface of the zirconia. This allows reaction sites to become "available" for interaction with zirconia primers.
Sandblasting alone, after the try-in and immediately before placement, is sufficient for freeing up the reaction sites. Therefore, for clinicians who will be performing the sandblasting themselves in the dental office, the use of a cleaning solution is optional but not necessary. However, if the clinician is requesting that the dental laboratory perform the sandblasting, then the restoration should be treated with a cleaning solution after the try-in, in order to facilitate the chemical interaction between the primer and the zirconia.
CONVENTIONAL CEMENTATION VS BONDING OF ZIRCONIA RESTORATIONS
No one specific universal protocol exists for the placement of zirconia restorations. The optimal means of treating both the zirconia and tooth surfaces before placement of the restorations depends on numerous factors, including the retentiveness of the preparation, the minimum occlusal thickness of the restoration, whether the zirconia is high-strength or translucent, and the nature of the conventional or resin-based cement used.
As mentioned earlier, the author recommends sandblasting of the intaglio surface of all zirconia restorations, and a zirconia primer (typically a phosphate ester such as 10-MDP) should be used after sandblasting when a resin-based cement is used. However, the use of a separate zirconia primer is in fact contraindicated or unnecessary with some materials. For example, when using a "bioactive" glass ionomer/calcium aluminate hybrid cement that is very hydrophilic in nature, the zirconia surface should be sandblasted but a zirconia primer such as 10-MDP should not be used, according to the manufacturer (Dr. Jesper Loof Tekn, CEO and Executive VP of Operations and Research and Development, Doxa Dental, personal communication). (A discussion of the reasons for this contraindication is beyond the scope of this article; for details on this topic, the reader is referred to reference 31.) Likewise, conventional glass ionomer cements and polycarboxylate cements do not require the use of a separate zirconia primer.
Resin-modified glass ionomer (RMGI) cements have become popular with many dental clinicians, as these cements have many positive attributes, including good physical properties, low solubility, low film thickness, fluoride release, low incidence of postoperative sensitivity, and a good long-term clinical track record. Perhaps the greatest clinical advantages of RMGI cements are the ease with which they are mixed, placed, and cleaned up. In the author's opinion, RMGI cements are among the best cementation options for high-strength zirconia, provided that the preparations have proper resistance and retention form and a minimum occlusal thickness of at least 1 mm.
It is important to note that while 1 mm is an adequate minimum occlusal thickness for a conventionally cemented 3 mol% yttria (high-strength) zirconia crown, most manufacturers recommend a minimal thickness threshold of 1.5 mm for conventionally cemented 5 mol% (translucent) zirconia.
For restorations with preparations that have an occlusal thickness of less than 1 mm, that do not have proper resistance and retention form, or where maximum adhesion is required (eg, the wings of a resin-bonded bridge), resin cement of some type should be utilized. This can take the form of a self-etching self-priming resin-based cement (eg, 3M™ RelyX™ Unicem, 3M ESPE; Maxcem Elite™, Kerr; TheraCem®, BISCO, Inc.; G-CEM™, GC America) or a resin-based cement used in tandem with a dentin bonding agent (eg, Duo-Link Universal™, BISCO, Inc.; 3M™ RelyX™ Universal Resin Cement, 3M ESPE; Multilink®Automix, Ivoclar). Resin-based cements are a better choice than RMGI and other conventional cements for bonding restorations in or on minimally retentive preparations, because they bond more predictably and durably to both zirconia and tooth tissue. Resin-based cements also allow better stress distribution when loaded, help prevent crack propagation, and optimize overall assembly strength, and may therefore be a better choice for translucent zirconia or zirconia restorations with minimal occlusal thickness.24 Disadvantages of resin-based cements are that they can present challenges in clean-up, are more technique sensitive, and involve extra steps when used in tandem with a dentin bonding agent. The latter disadvantage can be obviated with the use of dual-cure self-etching, self-priming resin cements, which are popular because they do not require a separate bonding agent. Nevertheless, the highest bond to tooth structure is achieved by resin cements when used in tandem with a separately placed bonding agent.32-34
CONCLUSION
A common misconception in the dental community is that "you cannot bond to zirconia." On the contrary, based on the requirements of the individual case and on clinician preferences, zirconia dental restorations can in fact be bonded or conventionally cemented with great predictability, using a combination of sandblasting, a phosphate ester primer such as 10-MDP, and an appropriate resin-based cement. As a general rule, the intaglio surface of all zirconia restorations should be air-particle-abraded and a zirconia primer should be used, although there are exceptions to this protocol and the use of a separate zirconia primer may be contraindicated or unnecessary with certain materials. For this reason, it is necessary that clinicians precisely follow manufacturer instructions and recommendations. It is the responsibility of all clinicians to be knowledgeable about the optimal cementation options and protocols for placing zirconia restorations, in order to achieve the best results for the patient.
References
1. Fischer J, Stawarczyk B, Hämmerle CH. Flexural strength of veneering ceramics for zirconia. J Dent. 2008;36(5):316-321.
2. Matsuzaki F, Sekine H, Honma S, et al. Translucency and flexural strength of monolithic translucent zirconia and porcelain-layered zirconia. Dent Mater J. 2015;34(6):910-917.
3. Kayahan ZO. Monolithic zirconia: a review of the literature. Biomedical Research. 2016;27(4):1427-1436.
4. Ritzberger C, Apel E, Höland W, Peschke A, Rheinberger VM. Properties and clinical application of three types of dental glass-ceramics and ceramics for CAD-CAM technologies. Materials (Basel). 2010;3(6):3700-3713.
5. Burgess JO, Janyavula S, Lawson NC, Lucas TJ, Cakir D. Enamel wear opposing polished and aged zirconia. Oper Dent. 2014:39(2):189-194.
6. Daou EE. Esthetic prosthetic restorations: reliability and effects on antagonist dentition. Open Dent J.2015;9:473-481.
7. Esquivel-Upshaw JF, Kim MJ, Hsu SM, et al. Randomized clinical study of wear of enamel antagonists against polished monolithic zirconia crowns. J Dent. 2018;68:19-27.
8. Terki R, Bertrand G, Aourag H, Coddet C. Cubic-to-tetragonal phase transition of HfO2 from computational study. Materials Letters. 2008;62(10-11)1484-1486.
9. Chevalier J, Gremillard L, Virkar AV, Clarke DR. The tetragonal-monoclinic transformation in zirconia: lessons learned and future trends. J Am Ceram Soc. 2009;92(9):1901-1920.
10. Della Bona A, Pecho OE, Alessandretti R. Zirconia as a dental biomaterial. Materials (Basel). 2015;8(8):4978-4991.
11. Della Bona A. Bonding to Ceramics: Scientific Evidences for Clinical Dentistry. Sao Paulo, Brazil: Artes Medicas; 2009.
12. Piconi C, Maccauro G, Pilloni L, Burger W, Muratori F, Richter HG. On the fracture of a zirconia ball head. J Mater Sci Mater Med. 2006;17(3):289-300.
13. Chevali J. What future for zirconia as a biomaterial? Biomaterials. 2006;27(4):535-543.
14. Brown SS, Green DD, Pezzotti G, Donaldson TK, Clarke IC. Possible triggers for phase transformation in zirconia hip balls. J Biomed Mater Res B Appl Biomater. 2008;85(2): 444-452.
15. Kwon SJ, Lawson NC, McLaren EE, Nejat AH, Burgess JO. Comparison of the mechanical properties of translucent zirconia and lithium disilicate. J Prosthet Dent. 2018;120(1):132-137.
16. Alex G. Universal adhesives: the next evolution in adhesive dentistry? J Compend Contin Educ Dent. 2015;36(1):15-28.
17. Kim B-K, Bae HE-K, Shim J-S, Lee K-W. The influence of ceramic surface treatments on the tensile bond strength of composite resin to all-ceramic coping materials. J Prosthet Dent. 2005;94:357-362.
18. Yi Y-, Ahn J-S, Park Y-J, et al. The effect of sandblasting and different primers on shear bond strength between yttria-tetragonal zirconia polycrystal ceramic and a self-adhesive resin cement. Oper Dent. 2015;40(1):63-71.
19. Barragan G, Chasqueira F, Arantes-Oliveria S, Portugal J. Ceramic repair: influence of chemical and mechanical surface conditioning on adhesion to zirconia. Oral Health Dent Manag. 2014;13(2)155-158.
20. Zandparsa R, Talua NA, Finkelman MD, Schaus SE. An in-vitro comparison of shear bond strength of zirconia to enamel using different surface treatments. J Prosthodont. 2014;23(2):117-123.
21. Zhang Y, Lawn BR, Rekow DE, Thompson VP. Effect of sandblasting on long-term performance of dental ceramics. J Biomed Mater Res B Appl Biomater. 2004;71(2):381-386.
22. Chintapalli RK, Marro FG, Jimenez-Pique E, Anglada M. Phase transformation and subsurface damage in 3Y-TZP after sandblasting. Dent Mater. 2013;29(5):566-572.
23. Hallmann L, Ulmer P, Reusser E, Hämmerle CHF. Effect of blasting pressure, abrasive particle size and grade on phase transformation and morphological change of dental zirconia surface. Surface and Coatings Technology.2012;206(19-20):4293-4302.
24. McLaren E, Burgess J, Brucia J. Cubic-containing zirconia: is adhesive or conventional cementation best? J Compend Contin Educ Dent. 2008;29(5):282-284.
25. Alnassar T, Ozer F, Chiche G, Blatz MB. Effect of different ceramic primers on shear bond strength of resin-modified glass ionomer cement to zirconia. J Adhes Sci Tech. 2016;30(22):2429-2438.
26. Nagaoka N, Yoshihara K, Feitosa VP, et al. Chemical interaction mechanism of 10-MDP with zirconia. Sci Rep. 2017;30(7):45563.
27. Xubiao L, Wu X, Reng Z, Min X, Xiao X, Luo J. Enhancement of phosphate adsorption on zirconium hydroxide by ammonium modification. Ind Eng Chem Res. 2017;56(34):9419-9428.
28. Chen L, Suh BI, Shen H. Minimize the contamination of zirconia restoration surface with saliva [abstract]. J Dent Res. 2013;92(spec iss A). Abstract 1654.
29. Khan AA, Al Kheraif AAA, Jamaluddin S, Elsharawy M, Divakar DD. Recent trends in surface treatment methods for bonding composite cement to zirconia: a review. J Adhes Dent.2017;19(1):
7-19.
30. Yang B, Lange-Jansen HC, Scharnberg M, et al. Influence of saliva contamination on zirconia ceramic bonding. Dent Mater. 2008;24(4):508-513.
31. Alex G. Zirconia - separating fact from fiction. Oral Health. July 2, 2019. https://www.oralhealthgroup.com/features/zirconia-separating-fact-from-fiction.
32. Barcellos DC, Batista GR, Silva MA, Rangel PM, Torres CR,
Fava M. Evaluation of bond strength of self-adhesive cements to dentin with or without application of adhesive systems. J Adhes Dent. 2011;13(3):261-265.
33. Chen C, He F, Burrow MF, Xie H. Bond strengths of two self-adhesive resin cements to dentin with different treatments. J Med Biol Eng. 2011;31(1):73-77.
34. Pisani-Proença J, Erhardt MC, Amaral R, Valandro LF, Bottino MA, Del Castillo-Salmeron R. Influence of different surface conditioning protocols on microtensile bond strength of self-adhesive resin cements to dentin. J Prosthet Dent. 2011;105(4):227-235.