You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
A Comprehensive Approach to Plan and Design the Emergence Profile Around Dental Implants
Giacomo Fabbri, DDS
To achieve favorable esthetic outcomes and maintain peri-implant health after implant placement, the appropriate design of the emergence profile of restorations is of paramount importance. The survival of dental implants is influenced by the achievement, integrity, and maintenance of osseointegration and mucointegration. The amount of peri-implant bone loss that occurs after implant placement and abutment connection has a major impact on the longevity of the dental implants, while a wide range of factors may contribute to the extent of early bone remodeling.1-4 Of these, implant connection, soft-tissue thickness, collar surface and macro-design, implant drilling protocol, surgical trauma, infection, the restorative materials that are used, abutment surface/design, and the abutment connection protocol appear to significantly influence initial bone remodeling at the time of implant placement.5-10
To attain ideal bone and soft tissue integration over the long-term, the most important aspects of the design and creation of the emergence profile around dental implants are:
• the morphology of the emergence contours11;
• the characteristics of the restorative materials-ie, their biocompatibility and their surface chemistry, including the decontamination and sterilization of the surfaces12-16; and
• the clinical approach that is used with the 3D implant placement and final abutment connection.17
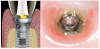
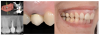
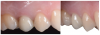
The emergence profile-also known as the implant restorative contour-can be defined as the three-dimensional (3D) shape of sub- and supramucosal contours as they emerge from the gingiva, and as such, is crucial to the esthetic appearance of the final restorations and to the maintenance of proper oral hygiene. The emergence profile can be divided into two zones: the subcritical and critical contours (Figure 1 and Figure 2). The subcritical contour, which is the deeper area of the emergence contour,18 is located immediately coronal to the implant platform and extends to the critical contour. The critical contour is located above the subcritical contour and extends to the free margin of peri-implant soft tissues up to 2.0 to 2.5 mm apically.19 The subcritical and critical contours determine the margin levels of the soft tissues and the architecture of the final restorations. Correct morphology of these contours is therefore crucial to achieve esthetic and stable results.
BIOLOGICAL WIDTH AND SOFT TISSUE HEALING
The development of a stable and healthy soft tissue barrier around dental implants is an indispensable prerequisite for the long-term success of implant-supported prostheses. A minimal biological width around dental implants is necessary to accommodate the soft tissue healing process, and the establishment of biological width may also contribute to early bone remodeling.2,8,20 Biological width is defined as the dimension of space occupied by healthy gingival tissues above the alveolar bone, measured from the bottom of the junctional epithelium to the tip of the alveolar bone.21,22 When this dimension is not present, bone resorption may occur to allow for an "appropriate biological dimension" of the peri-implant soft tissue barrier.
Healing of mucosa around a transmucosal implant component is challenging in the oral environment, owing to the continuous exposure of the mucosa to microorganisms. The rapid formation of an efficient mucointegration is therefore crucial to establish and maintain the health of peri-implant tissues. This mucosal complex is made up of a 2- to 2.5-mm-high epithelial apparatus (ie, the sulcular epithelium and junctional epithelium) and a 1- to 1.5-mm-high supracrestal integration of connective tissue.13,17,23-25 The junctional epithelium (JE) is nonkeratinized tissue and is thus an open system permeable in both directions even for large molecules. The inward pathway allows antigen and endotoxins from bacteria accumulated in the sulcus to reach the connective tissue, while via the outward pathway, sulcular fluid and defending cells migrate along the intercellular space to the sulcus. This phenomena, which increases during inflammation, represents the most important defense mechanism of peri-implant mucosa, with the JE acting as a seal/defense area. Because the turnover rate of the JE cells is exceptionally high26 and the area covered by daughter cells in the JE is greater than that through which JE cells desquamate, a strong funneling effect towards the sulcus occurs, enabling the JE to serve as the first barrier of antimicrobial defense. The exfoliation of JE cells also results in the effective removal of bacteria adhering to JE cells.19
Coronally, close to the sulcus, the JE is 15 to 30 cell layers wide and narrows towards the apical part of the tissue.19 The coronal two-thirds of the JE consist of two strata: the basal layer facing the connective tissue and the suprabasal layer facing the implant surface.23,24,26The apical third of the JE is made up of only two-cell layers and ends at its apical extremity in a one-cell layer of directly attached cells; the latter portion usually appears red in color, because the ultrathin thickness of the cell layer causes the underlying blood vessels to be visible (Figure 1).
The JE terminates at approximately 1 to 1.5 mm from the alveolar crest, partially covering the underlying supracrestal connective tissue. The JE protects the connective tissue and bone crest from oral pathogens coming from the sulcus. At the same time, the connective tissue provides epithelial tissues with nutrients coming from the vessels.25 The supracrestal connective tissue includes an avascular area of dense circular fibers near the implant surface; this avascular area is surrounded by a looser connective tissue with a 3D network of collagen fibers running in different directions.25,27 Peri-implant connective tissue is characterized by a low density of cells and blood vessels but is rich with collagen fibers and fibroblasts,27 which account for its usual whitish color (Figure 1). The orientation of connective fibers represents the main difference between periodontal and peri-implant tissues; in periodontal structures, the fibers run perpendicular to the long axis of the tooth, whereas in peri-implant tissues, fibers from the bone crest run parallel to the implant surface.17
CHARACTERISTICS OF THE RESTORATIVE MATERIALS
The characteristics of restorative materials that most greatly influence the integration and stability of the restorations are their biocompatibility and their surface chemistry, including the decontamination and sterilization of the surfaces of the materials. Here, we discuss the materials that are most appropriate for use in the submucosal portion of the soft tissues, in particular in the tissue subcritical area, where the prosthetic components interact with the connective tissue and the JE.
Similar to the emergence profile, the submucosal portion of the soft tissues can be divided into two areas: the tissue critical area (tCA) and the tissue subcritical area (tSA). In the tSA, it is necessary to use the dental materials that combine a high biocompatibility and adequate biomechanical qualities, in order to promote biological integration and at the same time reliable restoration. To date, the two most strategic materials that exhibit these features are titanium and zirconia,13,14 which should be thoroughly polished without any layer of glaze, ceramic, or stains. It has been shown that soft tissues cells interact differently on smooth and rough surfaces.6,14 Rough surfaces have been found to be favorable for cellular adhesion of fibroblasts28 but accumulate more subgingival plaque than smooth surfaces.29 A proper balance between roughness and complete smoothness is therefore ideal for achieving good cell adhesion while at the same time preventing accumulation of plaque. The ideal roughness for both titanium and zirconia ranges between 0.1 and 0.2 µm.28,30,31
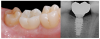
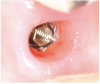
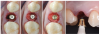
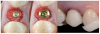
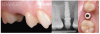
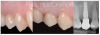
Tissue integration also depends on the surface properties of the materials in contact with peri-implant tissues, such as the surface energy and the decontamination and sterilization of the surfaces. Customized Computer-Aided Design/Computer-Aided Manufacturing (CAD/CAM) titanium and zirconia abutments may be fabricated using multiple technical procedures, all of which inevitably contaminate the surfaces of the materials, thus potentially compromising soft tissue integration and adherence.32 In vitro results have suggested that the decontamination of abutment surfaces is important for early fibroblast adherence and may improve the biological integration of peri-implant soft tissues.29 Ideally, abutments should have a surface that is completely decontaminated and sterilized, so as to allow for tight soft tissue adherence and integration in the subcritical and critical zones to support peri-implant tissues as well as create an effective soft-tissue seal/defense area against bacterial microorganisms. To achieve the best clinical performance in terms of soft tissue adherence, stock final abutments that are completely decontaminated and sterilized, with surface chemistry that boosts their biological response, should be the first choice (Figure 3 and Figure 4). These abutments are available in multiple heights, which should be selected based on the implant site, the vertical soft tissue thickness, and the esthetic expectations.
ABUTMENT MORPHOLOGY
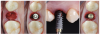
Abutment morphology is another important factor for bone and soft tissue integration and stability. As with the emergence profile and the soft tissues, the submucosal portion of the abutment can be divided into two areas: the abutment critical area (aCA) and the abutment subcritical area (aSA). Like the tissue subcritical area, the aSA is deeper, is located immediately coronal to the implant platform, and extends to the aCA.16 The aCA is located above the aSA and extends from the free margin of peri-implant soft tissues. The aCA determines the margin levels of the soft tissues and the architecture of final restorations.
The critical contour should be designed to correctly support the soft tissues, conditioning and scalloping the pink esthetics. The height of the aCA should range from 2 to 2.5 mm (Figure 2), depending on the esthetic demands of the individual clinical case. In this area, particularly in the most coronal portion, the cleanability of the abutment is crucial to reduce the risk of contamination, and therefore a thoroughly polished surface with mirror-like appearance is necessary. This is particularly important in patients with poor or suboptimal oral hygiene, with a history of periodontal disease, or who smoke.
Because the morphology of the aSA does not significantly influence the esthetic outcome, the subcritical contour should be designed primarily to preserve as much bone and soft tissue as possible and to achieve ideal soft tissue integration. The height of the aSA, which extends from the apical portion of the aCA to the implant platform, should range from 1 to 2.5 mm (Figure 2), but is highly variable in relation to the position of the implants in the corono-apical direction and to the thickness of crestal soft tissues.
CLINICAL APPROACH
One final important factor to consider in the creation of the emergence profile is the clinical approach used for the placement of the implants, particularly with regard to planning the vertical position of the implants in the corono-apical direction, and for implementing the abutment placement protocol. The achievement of proper implant restorative contours is highly dependent upon the 3D spatial location of the implants.33,34
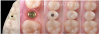
As discussed earlier, the biological width requires a minimal dimension to accommodate soft tissue healing. If the biological width is adequate, the crestal thickness of soft tissues should include the peri-implant soft tissue barrier or defense zone protecting the bone.8,13 A vertical distance of at least 3 to 3.5 mm (ie, a 2-mm-high epithelial apparatus and a 1.5-mm-high supracrestal connective tissue13,14,25,27,30,31,35) from the implant/abutment interface to the free mucosal margin should be considered to accommodate the biological width in the soft tissue thickness, avoid
crestal bone loss, and provide minimal vertical space to design the emergence profile (Figure 5 and Figure 6). This height should allow an ideal emergence profile morphology, avoiding excessively angled and steep horizontal components that could compromise oral hygiene maintenance. To achieve a gradual emergence profile in esthetic areas, the vertical distance may be increased up to 4.5 to 5.0 mm (Figure 2), particularly when the implants are placed slightly palatally. If the soft tissue thickness is not adequate, it will be necessary to increase it surgically (in particular in the frontal areas) or modify the vertical position of the fixture. Many studies have reported that adapting the vertical position of implants in relation to soft tissue thickness can prevent early implant surface exposure 8,10,13; however, this approach should be taken only when using implant systems with a stable conical connection and platform switching (Figure 1).6-10,29,33,34
Finally, once the proper 3D position of the implants has been identified, the abutment placement protocol to be used should be evaluated. The "one abutment/one time" approach4,6,8,17 allows soft tissues to adhere firmly onto the surface of titanium abutments, thereby improving soft tissue response and bone stability (Figure 4 and Figure 7 through Figure 14 ). This approach avoids multiple connections and disconnections at the bone interface and favors undisturbed bone and soft tissue healing.4-7
CONCLUSION
In the design and strategic development of the emergence profile of restorations, both prosthetic and surgical factors can significantly influence soft tissue integration, restoration stability, and early bone remodeling. The most important factors to consider in the design of the emergence profile are the characteristics and properties of the restorative materials, the morphology of the abutments, and the clinical approach used with 3D implant positioning and abutment placement. When selecting restorative materials, the clinician should consider the biocompatibility and surface properties of the materials, as these are essential for cell adhesion and proliferation onto the abutment surface. Correct morphology in the two zones of the emergence profile-ie, the critical and subcritical contours-is crucial for bone and soft tissue integration. Finally, the emergence profile should be evaluated in terms of the clinical approach necessary to accommodate the biological width and achieve an esthetic emergence profile, including determination of the correct vertical distance needed from the implant interface to the free mucosal margin. This design and approach to the emergence profile around dental implants will help achieve optimal soft tissue integration and a natural esthetic appearance, while preserving crestal bone from bacterial contamination and remodeling.
References
1.Fabbri G, Sorrentino R. A biologically driven concept to design
the emergence profile around dental implants: surgical and prosthetic considerations
to optimize hard and soft tissues integration. Int
J Periodontics Restorative Dent. 2021;41:doi: 1011607/prd.5063
2. Souza AB, Alshihri A, Kämmerer PW, Araújo MG, Gallucci GO. Histological and micro-T analysis of peri-implant soft and hard tissue healing on implants with different healing abutments configurations. Clin Oral Implants Res. 2018;29(10):1007-1015.
3. Albrektsson T, Dahlin C, Jemt T, Sennerby L, Turri A, Wennerberg A. Is marginal bone loss around oral implants the result of a provoked foreign body reaction? Clin Implant Dent Relat Res.2014;16(2):155-165.
4. Tallarico M, Caneva M, Meloni SM, Xhanari E, Covani U, Canullo L. Definitive abutments placed at implant insertion and never removed: is it an effective approach? A systematic review and meta-analysis of randomized controlled trials. J Oral Maxillofac Surg.2018;76(2):316-324.
5. Molina A, Sanz-Sánchez I, Martín C, Blanco J, Sanz M. The effect of one-time abutment placement on interproximal bone levels and peri-implant soft-tissues: a prospective randomized clinical trial.
Clin Oral Impl Res. 2017;28(4):443-452.
6. Canullo L, Annunziata M, Pesce P, Tommasato G, Nastri L, Guida L. Influence of abutment material and modifications on peri-implant soft-tissue attachment: a systematic review and meta-analysis of histological animal studies. J Prosthet Dent. 2020;125(3):426-436.
7. Fabbri G, Staas T, Linkevicius T, Valantiejiene V, González-Martin O, Rompen E. Clinical performance of a novel two-piece abutment concept: result from a prospective study with a 1-year follow-up.
J Clin Med. 2021;10(8):1594.
8. Gracis S. Prosthetic and biomechanical factors affecting bone remodeling around implants. Eur J Esthet Dent. 2013;8(2):314-333.
9. Linkevicius T, Puisys A, Steigmann M, Vindasiute E, Linkeviciene L. Influence of vertical soft tissue thickness on crestal bone changes around implant with platform switching: a comparative clinical study. Clin Implant Dent Relat Res. 2015:17(6):1228-1236.
10. Vervaeke S, Matthys C, Nassar R, Christiaens V, Cosyn J, De Bruyn H.
Adapting the vertical position of the implants with conical connection in relation to soft tissue thickness prevents early implant surface exposure: a 2-year prospective intra-subject comparison.
J Clin Periodontol. 2018;45(5):605-612.
11. Souza AB, Alshihri A, Kämmerer PW, Araújo MG, Gallucci GO. Histological and micro-CT analysis of peri-implant soft and hard tissue healing on implants with different healing abutments configurations. Clin Oral Implants Res.2018;29(10):1007-1015.
12. Welander M, Abrahamsson I, Berglundh T. The mucosal barrier at implant abutments of different materials. Clin Oral Implant Res.2008;19(7):635-641.
13. Sailer I, Philipp A, Zembic A, Pjetursson BE, Hämmerle CH, Zwahlen M. A systematic review of the performance of ceramic and metal implant abutments supporting fixed implant reconstructions. Clin Oral Implant Res. 2009;20(Suppl 4):4-31.
14. Linkevicius T, Vaitelis J. The effect of zirconia or titanium as abutment material on soft peri-implant tissues: a systematic review and meta-analysis. Clin Oral Implant Res. 2015;26(Suppl 11):139-147.
15. Zarone F, Di Mauro MI, Ausiello P, Ruggiero G, Sorrentino R. Current status on lithium disilicate and zirconia: a narrative review. BMC Oral Health. 2019;19(1):134.
16. Al Rezk F, Trimpou G, Lauer H-C, Weigl P, Krockow N. Response of soft tissue to different abutment materials with different surface topographies: a review of the literature. Gen Dent. 2018;66(1):18-25.
17. Tomasi C, Tessarolo F, Caola FTI, Wennström JL, Nollo G, Berglundh T. Morphogenesis of peri-implant mucosa revisited: an experimental study in humans. Clin Oral Implants Res. 2014;25(9):997-1003.
18. Schoenbaum TR, Kim YK, Khalifa F. Emergence contours for single-unit implant provisionals in the esthetic zone. Compend Contin Educ Dent. 2021;42(7):374-380.
19. Fabbri G, Sorrentino R. A biologically driven concept to design the emergence profile around dental implants: surgical and prosthetic considerations to optimize hard and soft tissue integration. Int J Periodontics Restorative Dent. 2021;41(6):913-921.]
20. Bishti S, Strub JR, Att W. Effect of the implant-abutment interface on peri-implant tissues: a systematic review. Acta Odontol Scand. 2014;72(1):13-25.
21. Nugala B, Kumar BBS, Sahitya S, Krishna PM. Biologic width and its importance in periodontal and restorative dentistry. J Conserv Dent. 2021;15(1):12-17.
22. Sharma A, Rahul GR, Poduval ST, Shetty K. Short clinical crowns (SCC) - treatment considerations and techniques. J Clin Exp Dent.2012;4(4):e230-e236.
23. Schupbach P, Glauser R. The defense architecture of the human periimplant mucosa: a histological study. J Prosthet Dent.2008;99(3):167.
24. Schwarz F, Mihatovic I, Becker J, Bormann KH, Keeve PL, Friedmann A. Histological evaluation of different abutments in the posterior maxilla and mandible: an experimental study in humans. J Clin Periodontol. 2013;40(8)807-815.
25. Shioya K, Sawada T, Miake Y, Inoue S, Yanagisawa T. Ultrastructural study of tissues surrounding replanted teeth and dental implants. Clin Oral Implants Res. 2009;20(3):299-305.
26. Skougaard MR. Cell renewal, with special reference to the gingival epithelium. Adv Oral Biol. 1970;4:261-288.
27. Berglundh T, Lindhe J, Jonsson K, Ericsson I. The topography of the vascular systems in the periodontal and peri-implant tissues in the dog. J Clin Periodontol. 1994;21(3):189-193.
28. Nothdurft FP, Fontana D, Ruppenthal S, et al. Differential behavior of fibroblasts and epithelial cells on structured implant abutment materials: a comparison of materials and surface topographies. Clin Implant Dent Relat Res. 2015;17(6):1237-1249.
29. Nakajima K, Odatsu T, Shinohara A, Baba K, Shibata Y, Sawase T. Effects of cleaning methods for custom abutment surfaces on gene expression of human gingival fibroblasts. J Oral Sci. 2017;59(4):533-539.
30. Al Rezk F, Trimpou G, Lauer HC, Weigl P, Krockow N. Response of soft tissue to different abutment materials with different surface topographies: a review of the literature. Gen Dent. 2018;66 (1):18-25.
31. Quirynen M, Bollen CM, Papaionnou W, Van Eldere J, van Steenberghe D. The influence of titanium abutment surface roughness on plaque accumulation and gingivitis: short-term observations. Int J Oral Maxillofac Implants. 1996;11(2):169-178.
32. Lüers S, Laub M, Jennissen HP. Protecting ultra- and hyperhydrophilic implant surfaces in dry state from loss of wettability. Curr Dir Biomed Eng. 2016;2:557-560.
33. Hermann F, Lerner H, Palti A. Factors influencing the preservation of the periimplant marginal bone. Implant Dent. 2007;16(2):165-175.
34. Canullo L, Fedele GR, Ianello G, Jepsen S. Platform switching and marginal bone-level alterations: the result of a randomized-controlled trial. Clin Oral Implants Res. 2010:21(1):115-121.
35. Zarone F, Di Mauro MI, Ausiello P, Ruggiero G, Sorrentino R. Current status on lithium disilicate and zirconia: a narrative review. BMC Oral Health. 2019;19(1):134.