You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
Like so many other professions and industries throughout the world, the dental profession was simply not prepared for the economic and health ramifications of the coronavirus disease 2019 (COVID-19) pandemic. Yet despite statewide closures and diminishing supply chains, oral healthcare professionals have risen to the challenge, as they have worked diligently over the past year to identify and effectively execute their unique role as both infection control specialists and patient advocates.
Transmission of any infectious disease occurs by a process known as the "chain of infection," which consists of a sequence of six specific events, or "links." For infection to develop, the following cascade of events must occur: infectious agent, reservoir, portal of exit, mode of transmission, portal of entry, and susceptible host (Figure 1). Each stage in the chain therefore also presents an opportunity to prevent the development and propagation of infection.
It is the responsibility of oral healthcare professionals to identify these various opportunities to "break the chain" of infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus.
This article will describe the chain of infection as it relates to the propagation of SARS-CoV-2, well as discuss the role of dental professionals in reducing the risk of infection to aid in the worldwide recovery from the COVID-19 pandemic.
INFECTIOUS AGENT
The first event in the chain of infection is the presence of an infectious agent, which may be a bacterium, protozoan, fungus, helminth, or virus.1 To understand the nature of the transmission of COVID-19, we must therefore first understand the infectious agent responsible for this disease.
The human coronavirus family is a collection of viruses ranging from those responsible for the common cold (HCOV-OC43, HCOV-229E, HCOV-NL63, and HCOV-HKU1) to those resulting in the severe acute respiratory syndrome outbreaks of 2002 (SARS-CoV), Middle East respiratory syndrome-related coronavirus (MERS-CoV), and currently, COVID-19 (the 2019 novel coronavirus, SARS-CoV-2).2
Although research is still underway to fully understand the SARS-CoV-2 virus, information about the virus particulates is clear. SARS-CoV-2 is termed "coronavirus" because of the spike glycoproteins that protrude from the viral envelope like the spikes of a crown (or, "corona") and encapsulate the body of the virus. The average diameter of the envelope of the virus is .08 μm, and each spike averages .02 μm in length, providing an average particulate diameter of approximately .12 μm.3 The size of this virus is of great concern for dental professionals and infection control specialists alike, as any particulate of this size is considered "ultra-fine," meaning that physical barriers of the body such as the nasal filtration system and the alveoli are susceptible to particulates of this size.3
Breaking the Chain: Infectious Agent
Currently, researchers are investigating the unique antiviral approaches for eradicating this virus. One approach that has demonstrated efficacy is the integration of an oxidation reaction.4 In terms of attenuating the human coronavirus, current research indicates that a targeted oxidation reaction provides an ideal antiviral activity.5 Oral rinsing medicaments such as chlorine dioxide,6 hydrogen peroxide, and povidone-iodine, as well as xylitol applied to the nasopharynx have been shown to provide efficacy in oxidation reactions.7
RESERVOIR
The next link in the chain of infection is the reservoir of the infection. The term reservoir loosely refers to the site where the infectious agent is able to grow and multiply, and is also used to describe an infected individual who harbors the virus. Reservoirs can be either apparent (symptomatic) or inapparent (asymptomatic) in nature.1 It has been theorized that SARS-CoV-2 may be a zoonotic virus originating in bats via the RaTG13 bat coronavirus, although the origin remains unknown.8 Because COVID-19 is an infection that can be spread from human to human via respiratory droplets, community standards for controlling both apparent and inapparent reservoirs have been instituted via legislation and public sector requirements.
Apparent (Symptomatic) Reservoirs
A reservoir that permits the growth and multiplication of a microorganism while demonstrating overt signs of such is termed a symptomatic or an "apparent reservoir." Individuals with symptomatic COVID-19 may experience one or more of the following symptoms: fever, dry cough, shortness of breath, malaise, dyspnea, and fatigue.9 Other signs and symptoms may include runny nose or congestion, muscle pain, pink eye, loss of smell or appetite, sore throat, and/or diarrhea or nausea.9
Additionally, some patients present with a phenomenon termed "COVID toes," in which red lesions are noted on the soles of the feet and rash enlargements on the toes, demonstrating a possible skin reaction caused by a small clot or microclot in the blood vessels of the toes.10
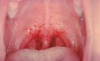
Systemic effects of COVID-19 may result in clogged alveoli and inflammation of the alveoli that can lead to pulmonary embolism, weakening of the heart muscle that can lead to arrhythmias and myocardial infarctions, and a widespread negative impact on the immune system.11 Finally, dental professionals have observed enanthem or a transient erythematic lesion in some patients, particularly noted in the junction between the hard and soft palate (but also sometimes observed across the buccal mucosa or under the tongue).12 This manifestation is often associated with the viruses responsible for coxsackie disease, measles, chicken pox,13 and now COVID-19 as well (Figure 2).
Breaking the Chain: Apparent Reservoirs
Dental practices across the United States have integrated the use of patient screening forms as an initial means of identifying symptomatic carriers of the SARS-CoV-2 virus. A patient may be asked "Have you experienced fever, cough, difficulty breathing, and/or fatigue in the past 4 weeks?" At many dental practices, a patient's temperature is taken when the patient first arrives at the office; a body temperature exceeding 100.4°F indicates that the patient is symptomatic. (Dental office employees' temperatures are also taken regularly.) Strategies to reduce the presence of symptomatic reservoirs in the dental practice also include discouraging these patients from scheduling elective or nonurgent procedures until they are no longer contagious. It should be noted that viral shedding can last up to 49 days in exceptional cases.14 For this reason, dental practices should critically evaluate their protocols regarding the "waiting period" before allowing patients who previously tested positive for COVID-19 into the office for elective or nonurgent procedures.
In the event that a patient with known or suspected COVID-19 positivity must receive urgent dental care, the Centers for Disease Control and Prevention (CDC) highly suggest that the dental practice take the following precautions: all healthcare professionals who are in the room with the patient should wear an N95 or higher-level respirator as well as eye protection, gloves, and a gown; the number of healthcare professionals present during the procedure should be limited to only those essential for patient care and procedure support; aerosol-generating procedures should ideally take place in an airborne infection isolation room (AIIR); and the room surfaces must be cleaned and disinfected promptly following the procedure.15
Inapparent Reservoirs
A reservoir that permits the growth and multiplication of a microorganism without demonstrating overt signs of such is termed an "inapparent reservoir." Persons with COVID-19 who are asymptomatic are inapparent reservoirs. These inapparent or asymptomatic reservoirs may actually be ideal candidates for the spread of infection because of the ability of the microorganism to easily and quickly spread without being readily identified and addressed.16 The asymptomatic nature or relatively non-noxious symptoms in some individuals, such as loss of taste or smell, create great concern within the dental community and across communities.
The average incubation period of COVID-19 is 5.2 days, with the highest viral loads detected soon after symptom onset and gradually decreasing to the detection limit at about day 21.17 Inferred data at this time suggest that infectiousness of SARS-CoV-2 starts from 2.3 days before the first symptom and peaks at 0.7 days prior to prodromal symptom onset.18 Consequently, concerns regarding the ability of the virus to spread via inapparent reservoirs are valid.
Further, it is possible that dental unit waterlines may also house and permit growth of the virus, particularly in settings where water is stagnant or water quality is poor. Finally, research from the SARS-CoV epidemic of 2003 indicated that the virus was also present in the urine and feces of infected individuals; therefore, waste water is another concerning source of potential survival for this virus.19 Studies are finding incredible similarities in the behavioral patterns of SARS-CoV-2 and its predecessor, SARS-CoV.20
Breaking the Chain: Inapparent (Asymptomatic) Reservoirs
Evaluation of potential asymptomatic dental patients begins via patient screening. At many dental practices, patients are asked if they have been in contact with any confirmed COVID-19-positive patients or persons who are self-isolating because of a determined risk for COVID-19. Patients are also asked if they are employed at a workplace that is considered high risk owing to routine close contact with many individuals. Based on the average incubation period of COVID-19, patients who have traveled outside of the United States or to a known hot spot or area of moderate to high community transmission rate should be advised not to visit the dental practice until 14 days have elapsed from the time of their return from travel.21
A condition termed "happy hypoxic" describes an individual who is experiencing severe deprivation of oxygen but is seemingly unaware of their condition.22 Silent hypoxia that progresses rapidly to respiratory failure may provide an explanation as to why some younger individuals with COVID-19 who were previously otherwise asymptomatic experience advanced signs and symptoms later in the course of the disease.23 Pulse oximeter readings are used to identify potential "happy hypoxic" cases. It is believed that silent hypoxia may indicate asymptomatic COVID-1924 (Table 1).
Treatment of the dental unit waterline should include flushing waterlines at the beginning of each day and after each patient visit, using water that meets EPA regulatory standards, testing the quality of the water based on manufacturer instructions to thus ensure a water test under 500 CFU/mL, following manufacturer directions regarding the use of a chemical shock within the waterlines, and ensuring that filters on ultrasonic units are replaced appropriately.25-27 Finally, as in the case of the SARS-CoV epidemic, it is possible that treatment of waste water may become a community public health initiative in the effort to fully eradicate the SARS-CoV-2 microorganism.28
PORTAL OF EXIT
Once a microorganism has had the opportunity to thrive within its chosen reservoir, it must leave the reservoir to continue to infect other hosts. A portal of exit permits the movement of the microorganism from one reservoir into a new host and can include (but is not limited to) the following tracts: respiratory, gastrointestinal, and urinary tracts, as well as blood and the skin and other membranes.
In the case of SARS-CoV-2, it has been noted that the portal of exit is via the respiratory tract, where the virus exits in the form of respiratory secretions (see "Mode of Transmission" section) and may also include the fecal-oral route, as SARS-CoV-2 has been detected in the fecal matter of infected individuals.28
Researchers are currently examining whether the dental unit waterline or the air port on the dental unit can also provide a means of exporting the virus.25
Breaking the Chain: Portal of Exit
One of the CDC's strongest initiatives to control the spread of infection is the recommendation for the widespread use of face coverings in public settings or when social distancing of 6 feet is not possible. Of note, this initiative aims to protect the general public not only by preventing the emission of respiratory secretions but also by limiting the potential inapparent
(asymptomatic) reservoirs that people may encounter. It should be acknowledged, however, that dental patients pose a unique challenge in that they cannot wear a face mask while receiving treatment. It is therefore imperative that the oral healthcare provider wear a personal protective face covering when evaluating the portal of entry.
MODE OF TRANSMISSION
The mode of transmission is the means by which the infectious agent moves between an existing reservoir and a new host. Modes of transmission are typically categorized as either direct (droplet) or indirect (airborne, vehicle-borne or vector-borne). SARS-CoV-2 is known to spread person-to-person via direct respiratory droplets in close person-to-person contact as well as by indirect airborne transmission via aerosols.29
Aerosols
Aerosols can be defined as respiratory secretions, ie, standard respiratory secretions (emitted during breathing or talking) or advanced respiratory droplets (emitted during sneezing or coughing). Of greater concern in dentistry are mixed aerosols-for example, those generated by dental equipment during direct patient care or via droplets entering the air components of handpiece turbines, for example. These aerosols comprise a combination of pooled saliva, gingival crevicular fluid, blood, pulverized biofilm or enamel, dentin and restorative materials, and respiratory secretions generated via clean or antimicrobial water spray at a high velocity.
Generation of potentially infectious sprays in the dental practice are loosely defined as either splatter or aerosols (Table 2).
Studies have found that if SARS-CoV-2 is able to be effectively aerosolized, it can remain suspended in aerosol for up to 3 hours, on copper surfaces for up to 9 hours, on cardboard surfaces for up to 24 hours, and on plastic and stainless steel surfaces for up to 72 hours.20 This has required a critical evaluation not only of the survival rate of SARS-CoV-2 in aerosol form, but also of how effective current aerosol mitigation strategies that are used during the dental appointment (eg, controls such as high-volume evacuation and use of a rubber dam) are in reducing the time that a particular virus can remain suspended in the air. Research has clarified that during dental procedures, the greatest concentration of particles is present at the end of the procedure, and a mean aerosol amount of .022 units is still present 2 hours after the procedure.30
As a result, researchers are continually evaluating the possibility that viral spreading may occur indirectly between two dental patients by way of exposure of aerosolized SARS-CoV-2. Over the past year, dental practices across the United States have been critically evaluating the production of aerosols in the operatory as well as exploring opportunities to mitigate the infection risk that these aerosols pose for patients and dental staff members. Of note, the ultrasonic scaler and high-speed handpieces produce more airborne contamination than any other instrument in dentistry,7 although nearly any procedure in which the clinician is working within mixed aerosols may generate some form of dental aerosols (Table 3).
Currently, there are no clear parameters for the virulence or the concentration of pathogenic microorganisms present in aerosols generated by dental equipment. For example, use of a sonic or ultrasonic scaler introduces aerosols during the dental procedure, yet it should be noted that these aerosols are encapsulated in water particulates that may greatly dilute the concentration of microorganisms.31
Researchers are now beginning to critically evaluate the virulence of dental aerosols to better understand the risks of respiratory-acquired infections.32
Breaking the Chain: Mode of Transmission
As standardized precautions, guidelines regarding aerosol-reduction controls for use during aerosol-generating procedures have been instituted.33 These controls include proper handling of dental equipment used in aerosol-generating procedures (including utilization of the proper inserts, tips, or burs, as well as selecting the lowest effective power setting and water flow necessary for the task), performing a preprocedural mouth rinse, and the use of high-volume evacuation systems during aerosol-generating procedures. In addition, dental professionals are evaluating the potential benefit that a laser bacterial reduction setting may offer for reducing subgingival microorganisms before dental procedures.34 Finally, the use of High Efficiency Particulate Air (HEPA)-approved filtration systems, proper ventilation systems, and the use of an upper-room ultraviolet germicidal irradiation system have been instituted to better control the presence of virulent aerosols in the dental operatory.
PORTAL OF ENTRY
Although the known portal of entry for SARS-CoV-2 has been in question, current CDC statements indicate that it appears to be mucosal membranes, particularly via the respiratory tract.35 Upon entering via a mucosal membrane, the virus must be able to enter a host, lock into a host cell, then replicate within its new host. Of note, the spike-like protein on the surface of SARS-CoV-2 binds into angiotensin converting enzyme 2 receptors; these receptors are readily present in many cell types and tissues, including those of the heart, blood vessels, kidneys, liver, and gastrointestinal tract, as well as within the epithelium in the nose, mouth, and lungs. Most notably, these receptors are highly expressed in salivary tissue.36,37As such, these receptor sites are ideal sites for viral replication because they act as a cellular doorway for the virus that causes COVID-19.
Breaking the Chain: Portal of Entry
Protecting the portal of entry (ie, mucosal membranes) of the host requires an understanding of engineering controls and the use of personal protective equipment. Specifically, measures aimed at protecting the mucosal membranes of potential new hosts have been critically evaluated. Dental practices have employed social distancing, plastic partitions, and touchless check-in and check-out systems to reduce the spread of infection in the administrative areas. Clinicians have used consistent eye protection and either an ASTM level III surgical mask with a face shield or an N95 (or higher) respirator (with an optional face shield).
Additional personal protective measures include but are not limited to: surgical caps, surgical gowns (Occupational Safety and Health Administration [OSHA] mandated), and shoe coverings. Hand hygiene has also become a great focus, as previous research studies indicate that before the pandemic, fewer than 25% of dental professionals routinely performed appropriate hand sanitization procedures.38 Hand hygiene aims to reduce the introduction of viral particulates into mucosal membranes by vehicle-borne transmission from the hands.1
SUSCEPTIBLE HOST
An encounter with the virus alone is not enough to permit the viral replication process; a host must be susceptible or vulnerable to the virus. Early in the pandemic, it was noted that susceptibility included but was not limited to advanced age (≥65 years old), heart disease, lung disease, diabetes mellitus, hypertension, and obesity.39,40 However, research is continuing to explore the possibility that individuals with other pre-existing conditions, such as income, education, and race, as well as oral disease, may either be more susceptible to acquiring the virus or more vulnerable to experiencing advanced signs and symptoms of the virus.41,42 It should be noted that, in addition to systemic conditions, oral biofilm and the subsequent inflammation that is caused by the biofilm may be key indicators in prolonging viral shedding in patients with COVID-19.43
Breaking the Chain: Susceptible Host
Immune-boosting strategies include attaining ample sleep, stress reduction, optimized nutrition, daily multivitamins and supplements, and reducing the risk of other immune-diminishing infections. Additional practices for improving respiratory health and thus reducing the risk of viral susceptibility are exercise to reduce the risk of acute respiratory distress syndrome,44 daily probiotics aimed to diversify the gut flora,45 receiving the flu vaccine,46 and ensuring ample amounts of fat-soluble vitamin D.47
CONCLUSION
Many dental professionals admit that the onset of the current global pandemic has been a catalyst for critically evaluating and reconstructing infection control standards across the dental industry. As the dental profession continues to evaluate the ways in which infection control standards are evolving amid the current global pandemic, it becomes increasingly imperative that current standards are not only met but that new standards are developed in tandem with the findings of peer-reviewed studies.
About the Author
Katrina M. Sanders, RDH, BSDH, MEd, RF
Clinical Liaison of Hygiene Excellence & Innovation, AZPerio, Phoenix, Arizona
References
1. Wilkins EM, Wyche CJ, Boyd LD. Clinical Practice of the Dental Hygienist. 12th ed. Philadelphia, PA: Wolters Kluwer; 2017.
2. Kaur N, Singh R, Dar Z, et al. Genetic comparison among various coronavirus strains for the identification of potential vaccine targets of SARS-CoV2. Infect Genet Evol. 2021;89:104490.
3. Neuman BW, Adair BD, Yoshioka C, et al. Supramolecular architec-
ture of severe acute respiratory syndrome coronavirus revealed by electron cryomicroscopy. J Virol. 2006;80(16):7918-7928.
4. Kirk-Bayley J, Sunkaraneni V, Challacombe S. The use of povidone iodine nasal spray and mouthwash during the current COVID-19 pandemic may reduce cross infection and protect healthcare workers. May 4, 2020. Available at https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3563092. Accessed July 16, 2021.
5. Miura T, Shibata T. Antiviral effect of chlorine dioxide against influenza virus and its application for infection control. The Open Antimicrobial Agents Journal. 2010;2:71-78.
6. Grootveld M, Silwood C, Gill D, Lynch E. Evidence for the microbicidal activity of a chlorine dioxide-containing oral rinse formulation in vivo. J Clin Dent. 2001;12(3):67-70.
7. Kirk-Bayley J, Sunkaraneni S, Challacombe S. The use of povidone iodine nasal spray and mouthwash during the current COVID-19 pandemic may reduce cross infection and protect healthcare workers. http://dx.doi.org/10.2139/ssrn.3563092. Published May 4, 2020. Accessed June 22, 2021.
8. Lau SKP, Luk HKH, Wong ACP, et al. Possible bat origin of se-
vere acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020;26(7):1542-1547.
9. Paules CI, Marston HD, Fauci AS. Coronavirus infections-more than just the common cold. JAMA. 2020; 323(8):707-708.
10. Hernandez C, Bruckner AL. Focus on "COVID toes."JAMA Dermatol.2020;156(9):1003.
11. Motta JDS Jr, Miggiolaro AFRDS, Nagashima S, et al. Mast cells
in alveolar septa of COVID-19 patients: a pathogenic pathway that may link interstitial edema to immunothrombosis. Front Immunol 2020;11:574862.
12. Jimenez-Cauhe J, Ortega-Quijano D, de Perosanz-Lobo D, et al. Enanthem in patients with COVID-19 and skin rash. JAMA Dermatol. 2020;156(10):1134-1136.
13. Cherry JD. Contemporary infectious exanthems. Clin Infect Dis.1993;16(2):199-205.
14. Tan L, Kang X, Zhang B, et al. A special case of COVID-19 with long
duration of viral shedding for 49 days. MedRxiv. doi: https://doi.org/10.1101/2020.03.22.20040071. Published March 27, 2020. Accessed June 22, 2021.
15. Centers for Disease Control and Prevention. Infection Control. IV:
Recommendations. Guideline for isolation precautions: preventing transmission of infectious agents in healthcare settings (2007). www.cdc.gov/infectioncontrol/guidelines/isolation/recommendations.html. Accessed June 22, 2021.
16. Qiu X., Nergiz AI, Maraolo AE, Bogoch II, Low N, Cevik M. Defining
the role of asymptomatic and pre-symptomatic SARS-CoV-2 transmission-a living systematic review. Clin Microbiol Infect. 2021;27(4):511-519.
17. Alene M, Yismaw L, Assemie MA, Ketema DB, Gietaneh W, Birhan TY. Serial interval and incubation period of COVID-19: a systematic review and meta-analysis. BMC Infect Dis. 2021;21(1):257.
18. Rabi FA, Al Zoubi MS, Kasasbeh GA, Salameh DM, Al-Nasser AD. SARS-CoV-2 and coronavirus disease 2019: what we know so far. Pathogens. 2020;9(3):231.
19. Wang X-W, Li J-S, Jin M, et al. Study on the resistance of severe
acute respiratory syndrome-associated coronavirus. J Virol Methods. 2005;126(1):171-177.
20. van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564-1567.
21. Alharbi A, Alharbi S, Alqaidi S. Guidelines for dental care provision
during the COVID-19 pandemic. Saudi Dent J. 2020;32(4):181-186.
22. Boal NS. Happy hypoxic. Am J Ophthalmol. 2020;220:A10-A11.
23. Wilkerson, RG, Adler, JD, Shah, NG, Brown, R. Silent hypoxia: a harbinger of clinical deterioration in patients with COVID-19. Am J Emerg Med. 2020;38(10):2243-e5-2243.e6.
24. Teo J. Early detection of silent hypoxia in Covid-19 pneu-
monia using smartphone pulse oximetry. J Med Syst. 2020:44(8): 134.
25. Alkhulaifi MM, Alotaibi DH, Alajlan H, Binshoail T. Assessment of
nosocomial bacterial contamination in dental unit waterlines: impact of flushing. Saudi Dent J. 2020;32(2):68-73.
26. O'Donnell MJ, Boyle MA, Russell RJ, Coleman DC. Man-
agement of dental unit waterline biofilms in the 21st century. Future Microbiol.2011;6(10):1209-1226.
27. Pankhurst CL, Coulter WA. Do contaminated dental unit
waterlines pose a risk of infection? J Dent. 2007;35(9):712-720.
28. Wang X, Li J-S, Jin M, et al. Study on the resistance of severe
acute respiratory syndrome-associated coronavirus. J Virol Methods. 2005;126(1-2):171-177.
29. Meselson M. Droplets and aerosols in the transmission of SARS-
CoV-2. N Engl J Med. 2020;382(21):2063.
30. Harrel SK, Molinari J. Aerosols and splatter in dentistry: a brief
review of the literature and infection control implications. J Am Dent Assoc.2004;135(4):429-437.
31. Wu M, Chang YC. COVID-19 and its implications in dental care management against bioaerosol transmission. J Dent Sci. 2020;15(3):367-368.
32. Mupparapu M. Aerosol reduction urgency in post-COVID-19 dental practice. Quintessence Int. 2020;51(7):525-526.
33. Virdi MK, Durman K, Deacon S. The debate: what are aerosol-
generating procedures in dentistry? A rapid review. JDR Clin Trans Res. 2021;6(2):115-127.
34. Moritz A, Schoop U, Goharkhay K, et al. Treatment of periodontal pockets with a diode laser. Lasers Surg Med. 1998;22(5):302-311.
35. Bar-On YM, Flamholz A, Phillips R, Milo R. SARS-CoV-2 (COVID-19) by the numbers. Elife. 2020:9:e57309.
36. Paizan MLM, Vilela-Martin JF. Is there an association between periodontitis and hypertension? Curr Cardiol Rev, 2014;10(4):355-361.
37. Aguilera EM, Suvan J, Buti J, et al. Periodontitis is associated with hypertension: a systematic review and meta-analysis. Cardiovasc Res. 2020;116(1):28-39.
38. Myers R, Larson E, Cheng B. Hand hygiene among general dental
practice dentists: a survey of knowledge, attitudes and practices. J Am Dent Assoc. 2008;139(7):948-957.
39. Maragakis L. Coronavirus and COVID-19: who is at higher risk? https://www.hopkinsmedicine.org/health/conditions-and-diseases/coronavirus/coronavirus-and-covid19-who-is-at-higher-risk. Updated June 25, 2020. Accessed June 22, 2021.
40. Garg S, Kim L, Whitaker M, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 - COVID-NET, 14 States, March 1-30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):458-464.
41. Wiemers EE, Abrahams S, AlFakhri M, Hotz VJ, Schoeni RF, Seltzer JA. Disparities in vulnerability to complications from COVID-19 arising from disparities in preexisting conditions in the United States. Res Soc Stratif Mobil. 2020;69:100553.
42. Marouf N, Cai W, Said KN, et al. Association between periodontitis and severity of COVID-19 infection: a case-control study. J Clin Periodontol. 2021;48(4):483-491.
43. Warabi Y, Tobisawa S, Kawazoe T, et al. Effects of oral care on prolonged viral shedding in coronavirus disease 2019 (COVID-19). Spec Care Dentist. 2020;40(5)470-474.
44. Chen P, Mao L, Nassis GP, et al. Coronavirus disease (COVID-19): the need to maintain regular physical activity while taking precautions. J Sport Health Sci. 2020;9(2):103-104.
45. Tapiovaara L, Pitkaranta A, Korpela R. Probiotics and the upper respiratory tract - a review. Pediatric Infect Dis. 2016;1:19.
46. Centers for Disease Control. Key facts about seasonal flu vaccine.
https://www.cdc.gov/flu/prevent/keyfacts.htm. Updated June 8, 2021. Accessed June 23, 2021.
47. Hughes DA, Norton R. Vitamin D and respiratory health. Clin Exp Immunol. 2009;158(1):20-25.