You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
Over the past two decades, mineral trioxide aggregate (MTA) has become one of the most widely studied endodontic materials.1-3 The trioxide aggregate in MTA consists of calcium, aluminum, and selenium. MTA has several desirable properties in terms of its biocompatibility, bioactivity, hydrophilicity, radiopacity, sealing ability, and low solubility. The most important of these properties in dentistry are its biocompatibility and sealing ability. High biocompatibility encourages optimal healing responses. This has been observed histologically with the formation of new cementum in periradicular tissue areas and a low inflammatory response with bridge formation in the pulp space.4,5 The sealing that is achieved is due to the material’s expansion and contraction properties being very similar to dentin, which results in high resistance to both marginal leakage and to bacterial migration into the root canal system. A stable barrier to bacterial and fluid leakage is one of the key factors that facilitates clinical success.
A very practical advantage of MTA is that, unlike many other dental materials, it sets in the moist environment that is omnipresent in dentistry. When in contact with moisture, the material’s main component, which is calcium oxide, converts into calcium hydroxide, which many clinicians are familiar with.6 This conversion results in a high pH microenvironment, which has beneficial antibacterial effects. Unlike calcium hydroxide, however, MTA has very low solubility and maintains its physical integrity after placement.
MTA materials are derived from a Portland cement parent compound. Although these compounds are similar in some respects, Portland cement and MTA are not identical.7 MTA products undergo additional processing and purification. When compared to Portland cements MTA products have a smaller mean particle size and contain fewer toxic heavy metals.8
Historical Perspective
MTA was first introduced in the dental literature in 1993 and received Food and Drug Administration (FDA) approval in 1998.9,10 In 1999 ProRoot® MTA (DENTSPLY Tulsa Dental Specialties, www.tulsadentalspecialties.com) was the first commercially available MTA product to be launched in the United States. MTA Angelus® (Angelus, www.angelusdental.com / Clinician’ Choice Dental Products Inc., www.clinicianschoice.com) was launched in Brazil in 2001 and received FDA approval in 2011, making it available in the United States. (Author’s note: A new product said to be comparable to MTA Angelus is Endocem MTA, which is manufactured in South Korea by Maruchi Co. Ltd. [www.endocem.com]. The author is not aware of it being available in North America or of any research on it done in North America.)
MTA Angelus exhibits a reduced setting time, is sold in containers that permit more controlled dispensing, and possesses the same desirable properties as traditional MTA.6,11-13 While the original MTA product is sold in single-use packets, the newer MTA Angelus is packaged in air-tight bottles that allow practitioners to dispense a small volume of powder and reseal the remainder of the product in its original container for future use. Traditional MTA takes about 2 to 3 hours to set. MTA Angelus sets within 15 minutes of being prepared. The decreased setting time is sometimes desirable as clinicians can ensure the material is set at the time of placement and can proceed with their restorative procedures without being concerned about MTA washout. This reduced setting time is a result of a lower concentration of calcium sulfate, which is the substance responsible for the longer setting time in the original formulation.
MTA comes in grey and white versions. The first MTA products were grey, and most of the initial research was done on this formulation. Due to staining concerns that were reported when MTA residues were left in the clinical crown, the white version of MTA was introduced to the market in 2002.7 White MTA has shown a decreased potential for staining but clinicians should still be diligent in removing all traces of it prior to restoring the coronal access of teeth in the esthetic zone.14 The difference between the two colors is mostly due to a decrease in the concentrations of iron, aluminum, and magnesium oxides in white MTA.7,15 The major difference is in the relative proportion of iron oxide where white MTA was found to have 90.8% less when compared to the original grey MTA variety.15 Even with these modifications, white MTA still possesses similar properties to grey MTA cement.11,16,17
When first introduced, clinicians had difficulty handling MTA due to its wet sandlike consistency, which was unlike most other conventional dental materials. Following the introduction of several customized application devices, the handling and application of this material has become more predictable.
Clinical Applications
More than 24 million endodontic procedures are performed in the United States on an annual basis, with 5.5% of these procedures being advanced treatments such as periapical microsurgeries, perforation repairs, and apexification treatments.18 All of these endodontic procedures and some operative procedures have greatly benefitted from the availability of MTA, and they are discussed here in turn.
Pulp Capping
Pulpal exposures are sometimes inevitable when addressing large carious lesions. While some clinicians are hesitant to perform direct pulp capping procedures due to documented unpredictability as a definitive treatment option, MTA may help to improve the outcome of this treatment in the near future. MTA has the advantage of being less soluble than calcium hydroxide and offers an enhanced seal due to its setting expansion, which hermetically seals the pulp space, preventing bacterial contamination from the outside. Studies have shown that in asymptomatic cases or in cases with reversible pulpitis (where the infection has not spread into the pulp chamber proper), MTA pulp capping can serve as a viable treatment option.19,20 Histological studies have also shown less inflammation and more dentinal bridging when MTA is placed compared to conventional pulp capping with calcium hydroxide.5 In pulp capping, the rapid 15-minute set of MTA Angelus allows the final restoration to be placed without delay and in direct contact with the set MTA.
Vital Pulp Therapy (Pulpotomy and Apexogenesis)
In cases of irreversible pulpitis where bacteria have invaded the pulp chamber, a pulpotomy procedure can sometimes be considered.21-23 This procedure is also termed apexogenesis because its ultimate goal is to facilitate the complete formation of the apex and root. This procedure is carried out in immature teeth with incomplete root formation that contain vital pulp tissue. The radicular pulp, which is considered to be relatively free of inflammation, is retained. When this is done, at a histological level odontoblasts will differentiate, dentin will continue to be laid down, and root development should continue. This will result in thickening of the root walls, which reduces the risk of root fracture, and apical closure should occur (apexogenesis), forming a natural apical constriction that would facilitate any future root canal obturation procedures.
Clinical procedure: Once in the pulp chamber, clinicians should use a diamond bur, because it will cauterize the tissue and minimize the bleeding. After this is done, the area should be disinfected with an antimicrobial agent (sodium hypochlorite or chlorhexidine), followed by a saline rinse. Hemostasis is obtained with light pressure from a damp cotton pellet. The pellet is removed after 2 or 3 minutes, and the area is then ready to be filled with MTA.
Apexification
The treatment of a necrotic pulp in an immature root has long presented a challenge to clinicians due to the lack of an apical stop. This has classically been addressed with long-term calcium hydroxide treatment, which may require several years of treatment time, involve multiple visits, and, theoretically at least, increase the fracture potential of the root involved.24 MTA has become an excellent predictable alternative to address these issues by creating a biocompatible apical plug in a single visit.25,26
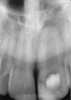
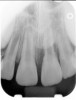
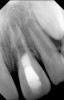
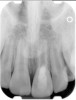
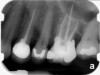
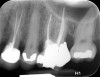
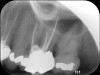
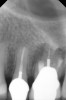
Clinical procedure: If apical bone loss is present (Figure 1) a collagen/gelatin sponge (eg, Gelfoam®, Pfizer Inc., www.pfizer.com) can be placed apically so that the MTA can be delivered to the desired working length. (Any other surgical resorbable sponge would also work, such as OraPlug® [Salvin Dental Specialties, www.salvin.com], Surgifoam® [Midwest Dental, www.mwdental.com], or Surgispon® [Aegis Lifesciences, www.surgispon.com]). This is done by taking a small piece (2 mm x 2 mm) of the resorbable sponge and pushing it down to and through the root apex with an endodontic file. Once this is done, MTA is packed down the canal with a custom-fitted cone. The clinician can use a rubber stopper on the gutta-percha cone to know the exact length of MTA placed in the apical third (Figure 2). Once the apical third is sealed with 3 mm to 5 mm of MTA, the remaining coronal canal space can be back-filled using a warm gutta-percha technique (Figure 3).
Regeneration
The treatment of a necrotic pulp in an immature root with very thin walls is problematic due to the high potential for root fracture. Regeneration of the dentin–pulp complex is a contemporary approach that involves disinfecting the root canal system with a triple antibiotic paste followed by tissue repair and regeneration.27,28 This should allow continued thickening of the lateral dentinal walls through deposition of new dentin-like hard tissue.27,28 More research is still needed to precisely assess if and how this newly deposited dentin-like hard tissue strengthens the pre-existing thin root.
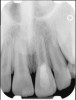
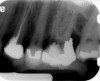
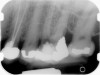
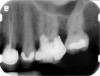
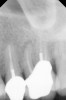
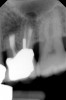
Clinical procedure: Regeneration of the endodontic pulp space is indicated for cases with very thin dentinal walls and an open apex that is more than 1 mm in diameter radiographically (Figure 4). Disinfection of the root canal system is performed using sodium hypochlorite irrigation followed by a triple antibiotic paste dressing that is left in place for 1 week. At the second visit, ethylenediaminetetraacetic acid (EDTA) is used to condition the dentin walls, which results in the release of growth factors, and bleeding is stimulated in the periapical tissues (where stem cells are located), with the aim of filling the pulp space with a stable blood clot, which would serve as the scaffold. MTA is then placed at the canal orifice in contact with the clot to protect it from coronal microleakage (Figure 5 and Figure 6). In time, the clot should be replaced with a reparative tissue of variable composition, and the root walls should continue to thicken due to the deposition of a dentin-like material on the pre-existing root dentin27,28 (Figure 7 and Figure 8).
Root Perforation
Perforations are generally the result of iatrogenic conditions in which a communication between the pulp canal and the periradicular tissue occurs during either access preparation or canal shaping procedures. Perforations can also happen in cases of internal root resorption, where the entire thickness of the root becomes affected by the resorptive process. Because of its excellent sealing ability and biocompatibility, MTA has been used to repair root perforations with predictable results.29,30
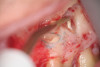
Clinical procedure: Once a perforation occurs, the extent of the perforation must be assessed. If there is an adjacent bony defect, the bony defect should first be filled with an osteoconductive or osteoinductive material. This can be done with a bone graft, calcium sulfate, or collagen/gelatin sponge. The dentinal portion of the tooth that has been perforated is then restored with MTA (Figure 9 through Figure 14).
Root-End Filling
A root-end filling (also known as a retrofilling) is performed when an extraradicular microsurgical approach is needed to address endodontic pathology. Most cases treated surgically cannot be predictably treated by orthograde conventional root canal methods due to either complex canal anatomy or iatrogenic misadventures during root canal treatments.31,32 MTA exhibits excellent physical sealing properties, and, furthermore, an additional biological seal is obtained by the proliferation of cells directly on the cementum during the healing process.4,32
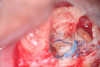
Clinical procedure: In the case of a root-end filling (Figure 15), once the apical 3 mm of the root has been resected (Figure 16) the canal system is then opened and cleaned with surgical ultrasonic tips to create the retro-preparation (Figure 17). After this is completed, the retro-preparation is dried and MTA is then placed and condensed in that space creating the retro-filling (Figure 18 through Figure 20).
Conclusion
Scientific research has demonstrated the effectiveness of traditional MTA when used in a range of endodontic procedures. The history, chemical composition, and clinical applications of MTA have been discussed in this review article. When considering sealing effectiveness and biocompatibility, MTA is unique and has advantages compared to other dental materials. With the recent introduction of a fast-setting MTA, which also offers excellent handling properties, MTA-based endodontic products are likely to remain at the heart of good dental practice for many years to come.
Disclosure
Dr. Tawil teaches continuing education courses sponsored by Clinician's Choice Dental Products Inc. but has no financial association with any of the company's products. The authors had no other disclosures to report.
About the Authors
Peter Z. Tawil, DMD, MS, FRCD(C), Dipl. ABE;
Jacob and Charlotte Freedland Term Professor
Endodontics
University of North Carolina School of Dentistry
Chapel Hill, North Carolina
Private Practice
Chapel Hill, North Carolina
Derek J. Duggan, BDentSc, MS, Dipl. ABE
Preclinical Endodontic Course Director
Endodontics
University of North Carolina School of Dentistry
Chapel Hill, North Carolina
Private Practice
Chapel Hill, North Carolina
Johnah C. Galicia, DMD, MS, PhD
Assistant Professor, Department of Endodontics, and Endodontist
Dental Faculty Practice
University of the Pacific Arthur A. Dugoni School of Dentistry
San Francisco, California
Queries to the author regarding this course may be submitted to authorqueries@aegiscomm.com.
References
1. Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review–Part I: chemical, physical, and antibacterial properties. J Endod. 2010;36(1):16-27.
2. Torabinejad M, Parirokh M. Mineral trioxide aggregate: a comprehensive literature review–Part II: leakage and biocompatibility investigations. J Endod. 2010;36(2):190-202.
3. Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review–Part III: clinical applications, drawbacks, and mechanism of action. J Endod. 2010;36(3):400-413.
4. Tawil PZ, Trope M, Curran AE, et al. Periapical microsurgery: an in vivo evaluation of endodontic root-end filling materials. J Endod. 2009;35(3):357-362.
5. Faraco IM Jr, Holland R. Response of the pulp of dogs to capping with mineral trioxide aggregate or a calcium hydroxide cement. Dent Traumatol. 2001;17(4):163-166.
6. Duarte MA, Demarchi AC, Yamashita JC, et al. Ph and calcium ion release of 2 root-end filling materials. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95(3):345-347.
7. Dammaschke T, Gerth HU, Züchner H, Schäfer E. Chemical and physical surface and bulk material characterization of white ProRoot MTA and two Portland cements. Dent Mater. 2005;21(8):731-738.
8. Islam I, Chng HK, Yap AU. Comparison of the physical and mechanical properties of MTA and portland cement. J Endod. 2006;32(3):193-197.
9. Lee SJ, Monsef M, Torabinejad M. Sealing ability of mineral trioxide aggregate for repair of lateral root perforations. J Endod. 1993;19(11)541-544.
10. Schmitt D, Lee J, Bogen G. Multifaceted use of ProRoot MTA root canal repair material. Pediatr Dent. 2001;23(4):326-330.
11. Menezes R, Bramante CM, Letra A, et al. Histologic evaluation of pulpotomies in dog using two types of mineral trioxide aggregate and regular and white Portland cements as wound dressings. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98(3):376-379.
12. Koulaouzidou EA, Economides N, Beltes P, et al. In vitro evaluation of the cytotoxicity of ProRoot MTA and MTA Angelus. J Oral Sci. 2008;50(4):397-402.
13. Lolayekar N, Bhat SS, Hegde S. Sealing ability of ProRoot MTA and MTA-Angelus simulating a one-step apical barrier technique–an in vitro study. J Clin Pediatr Dent. 2009;33(4):305-310.
14. Ioannidis K, Mistakidis I, Beltes P, Karagiannis V. Spectrophotometric analysis of coronal discolouration induced by grey and white MTA. Int Endod J. 2013;46(2):137-144.
15. Asgary S, Parirokh M, Eghbal MJ, Brink F. Chemical differences between white and gray mineral trioxide aggregate. J Endod. 2005;31(2):101-103.
16. Frenkel G, Kaufman A, Ashkenazi M. Clinical and radiographic outcomes of pulpotomized primary molars treated with white or gray mineral trioxide aggregate and ferric sulfate–long-term follow-up. J Clin Pediatr Dent. 2012;37(2):137-141.
17. Eskandarizadeh A, Shahpasandzadeh MH, Shahpasandzadeh M, et al. A comparative study on dental pulp response to calcium hydroxide, white and grey mineral trioxide aggregate as pulp capping agents. J Conserv Dent. 2011;14(4):351-355.
18. Nash KD, Brown LJ, Hicks ML. Private practicing endodontists: production of endodontic services and implications for workforce policy. J Endod. 2002;28(10):699-705.
19. Farsi N, Alamoudi N, Balto K, Al Mushayt A. Clinical assessment of mineral trioxide aggregate (MTA) as direct pulp capping in young permanent teeth. J Clin Pediatr Dent. 2006;31(2):72-76.
20. Bogen G, Kim JS, Bakland LK. Direct pulp capping with mineral trioxide aggregate: an observational study. J Am Dent Assoc. 2008;139(3):305-315.
21. Camp JH. Diagnosis dilemmas in vital pulp therapy: treatment for the toothache is changing, especially in young, immature teeth. J Endod. 2008;34(7 suppl):S6-S12.
22. Witherspoon DE. Vital pulp therapy with new materials: new directions and treatment perspectives–permanent teeth. J Endod. 2008;34(7 suppl):S25-28.
23. Barrieshi-Nusair KM, Qudeimat MA. A prospective clinical study of mineral trioxide aggregate for partial pulpotomy in cariously exposed permanent teeth. J Endod. 2006;32(8):731-735.
24. Andreasen JO, Farik B, Munksgaard EC. Long-term calcium hydroxide as a root canal dressing may increase risk of root fracture. Dent Traumatol. 2002;18(3):134-137.
25. Witherspoon DE, Ham K. One-visit apexification: technique for inducing root-end barrier formation in apical closures. Pract Proced Aesthet Dent. 2001;13(6):455-60.
26. Witherspoon DE, Small JC, Regan JD, Nunn M. Retrospective analysis of open apex teeth obturated with mineral trioxide aggregate. J Endod. 2008;34(10):1171-1176.
27. Banchs F, Trope M. Revascularization of immature permanent teeth with apical periodontitis: new treatment protocol? J Endod. 2004;30(4):196-200.
28. Miller EK, Lee JY, Tawil PZ, et al. Emerging therapies for the management of traumatized immature permanent incisors. Pediatr Dent. 2012;34(1):66-69.
29. Mente J, Leo M, Panagidis D, et al. Treatment outcome of mineral trioxide aggregate: repair of root perforations-long-term results. J Endod. 2014;40(6):790-796.
30. Main C, Mirzayan N, Shabahang S, Torabinejad M. Repair of root perforations using mineral trioxide aggregate: a long-term study. J Endod. 2004;30(2):80-83.
31. Chong BS. Managing Endodontic Failure in Practice. Chicago, IL: Quintessence Publishing Co.; 2004.
32. Kim S, Kratchman S. Modern endodontic surgery concepts and practice: a review. J Endod. 2006;32(7):601-623.