You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
Sjögren’s syndrome (SS) is a multisystem autoimmune disease characterized by chronic inflammation of exocrine glands, mainly the lacrimal and salivary glands. This commonly results in the development of xerophthalmia (dry eyes) and xerostomia.1-3 It can occur as a primary entity (primary SS) defined as the sicca syndrome accompanied by salivary swelling or in association with several other autoimmune diseases (secondary SS).1,4
Clinical features can involve the skin, eyes, oral cavity, salivary glands, and systems, including musculoskeletal, pulmonary, gastrointestinal, renal, neurologic, and hematologic. For this reason, making the diagnosis can be difficult.1,3-5 To improve accuracy, the diagnostic criteria should encompass the five objective tests/items that have a weight/score6: labial salivary gland with focal lymphocytic sialadenitis and focus score of ≥ 1 foci/4 mm2 (weight/score, 3); anti-SSA/Ro positive (weight/score, 3); ocular staining score ≥ 5 (or van Bijsterveld score ≥ 4) in at least one eye (weight/score, 1); Schirmer’s test ≤ 5 mm/5 minutes in at least one eye (weight/score, 1); unstimulated whole saliva flow rate ≤ 0.1 ml/minute (weight/score, 1). The individual is classified as having primary SS if he or she has a total score of ≥ 4, derived from the sum of the weights assigned to each positive test/item. These criteria sets help establish an early and accurate diagnosis of SS and are well suited as entry criteria for clinical trials and studies.4,6,7
Clinicians should be cognizant of the clinical oral features typical in SS, such as caries, premature teeth loss, loss of filiform papillae of the tongue, angular cheilitis, swelling of the parotid, and/or sublingual swelling that may appear before the onset of symptomatic xerostomia.8 By being aware of these signs, clinicians may be the first to recognize early manifestation of the disease and may help alleviate troublesome symptoms. This article presents a case report of a patient who received a diagnosis of SS. It highlights the combination of clinical features, laboratory investigations (serum and immunologic examinations), lower lip biopsy, and imaging findings (sialography and ultrasonography), which, taken together, improve diagnostic accuracy. The role of the clinician in the early diagnosis of SS and recommendations for management are also presented.
Case Report
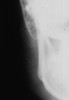
A 23-year-old woman presented with painful swelling in the parotid region. She also reported recurrent parotid gland swelling for the past 2 years that was satisfactorily treated with nonsteroidal anti-inflammatory drugs (NSAIDs) prescribed by the physician (Figure 1). A clinical examination disclosed a bilateral swelling of the parotid gland, predominantly on the right side, with associated xerostomia and xerophthalmia. The patient also reported photophobia (light sensitivity), foreign body sensation, and urticarial (hives) episodes. Additionally, she reported arthritis of the wrists and metacarpophalangeal and interphalangeal joints.
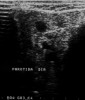
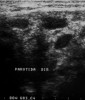
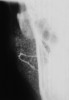
Imaging analyses, including ultrasonography and sialography, of the parotid glands were performed. Ultrasonographic features of the cervical region did not show any alterations of the submandibular and left parotid glands (Figure 2 through Figure 5). However, the right parotid gland demonstrated an inhomogeneous structure of the gland with scattered, multiple, small, oval, hypoechoic, or anechoic areas, usually well defined, and increased parenchymal blood flow. Sialographic examination of the right parotid gland showed a snowflake-like form and Christmas-tree pattern and evidence of sialoangiectasia without any changes of duct anatomy (Figure 6). Even after the salivary stimulation, the duct system was kept filled by the contrast fluid that again highlighted the sialectatic changes (Figure 7).
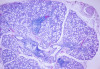
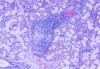
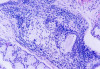
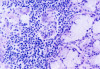
The laboratory studies showed hemoglobin, 13.1g/dL; white blood cells, 5140/mm3; and platelets, 165,000/mm3. A serum sample was tested for the presence of antinuclear antibodies (ANA) (indirect immunofluorescence using HEp-2 cell lines), anti-SSA and anti-SSB antibodies (enzyme-linked immunosorbent assay), and rheumatoid factor (nephelometric method). The results demonstrated a dotted pattern for the ANA test (1:320), positive results for anti-SSA test and negative findings for anti-SSB, and the rheumatoid factor was elevated at 80.5 µL/mL (normal, -20). Minor salivary gland biopsy revealed a focal periductal lymphocytic infiltrate, which, taken together with the other diagnostic test findings, confirmed the SS diagnosis (Figure 8 through Figure 11).
The evidence of ocular involvement was obtained using the Schirmer’s test (5 mm in 5 minutes, performed without anesthesia) and was positive for both eyes. Findings from minor salivary gland biopsy revealed a focal periductal lymphocytic infiltrate, which also helped confirm the diagnosis.
Discussion
SS affects women disproportionately, with a female:male ratio of 9:1, and may occur at any age but typically has its onset in the fourth to sixth decades of life.1,4,9 In the present case, the patient was given a diagnosis of primary SS during her third decade of life, 2 years after the start of the symptoms, which is earlier than 3 to 11 years reported by al-Hashimi.2 Because the patient was beginning studies in dentistry, she wanted to investigate the reasons for her painful parotid swelling, which accounts for the early diagnosis.
Typically, the wide variety of symptoms and clinical manifestations make SS underrecognized and, consequently, misdiagnosed. The most common early symptoms of dryness are considered minor or vague or mimic those of other diseases, such as amyloidosis, diabetes mellitus, sarcoidosis, viral infections, trauma, or irradiation. They may be psychogenic or medication induced.10 Consequently, its diagnosis is typically attained years after the onset of symptoms.4 Moreover, symptoms of SS may not always be present concurrently, which leads to dentists and physicians treating symptoms in isolation. An early and accurate diagnosis of SS can help provide symptom relief in the patient and help prevent or ensure timely treatment of many complications associated with the disease, such as malignant lymphoma.10
Many systemic and oral complications are associated with SS, which complicates timely diagnosis and management. The constant feeling of xerostomia is a result of the reduction in salivary flow, and the subsequent loss of the lubricating, buffering, and antimicrobial capacities can give rise to dysarthria and dysphagia.11 SS may also be associated with an increased frequency of dental caries, which tends to involve smooth surfaces and areas otherwise not prone to caries, such as the lower incisor region and roots. The condition may also increase the risk for candidosis (which may cause a burning sensation or mucosal erythema), angular stomatitis (angular cheilitis), acute bacterial sialadenitis, and other oral conditions.1,4,12 Although decreased salivary flow may lead to increased risk for periodontal disease, the association of both conditions represents a controversial topic in patients with SS.12-14 One investigator found that patients with SS had better oral hygiene, and no significant difference was detected in the periodontal status of SS patients compared with controls who had subjective xerostomia.12 Other investigators have noted that patients with SS seem to be at higher risk of having alveolar bone loss.13,14
Assessing available therapies of xerostomia has been difficult partly because of a lack of well-designed randomized, controlled clinical trials and insufficient diagnostic criteria for such disorders.11,15 According to Soto-Rojas and Kraus,16 treatment of oral manifestations remains empiric and symptomatic. Traditionally, treatment of xerostomia has focused on palliative measures with the use of stimulation of saliva or salivary substitutes along with therapy to create a remineralizing effect on dentin and enamel and provide long-lasting relief of the oral symptoms of xerostomia.12,15 However, in the present case, the patient’s acceptance of the salivary substitute was not good because it provided only insignificant and short-lived relief. Thus, therapy with sugarless chewing-gum exercises to stimulate saliva production was advised, which is preferred because of the protective effects of saliva.16 Although the patient had a normal periodontium and no dental caries, the dental team emphasized to her the importance of and need for general personal oral hygiene. Table 1 summarizes the principal recommendations of oral care in patients with xerostomia according to the Sjögren’s Syndrome Foundation Clinical Practice Guidelines Committee and other authors.15,17,18
Another common oral manifestation of this condition is salivary gland swelling, which occurs in as many as 30% of patients with SS during their illness, and the parotid gland is most commonly involved. The glands are typically firm and nontender, an important trait that differentiates SS from endocrine and metabolic disorders and viral infections.10,19 Persistent swelling should be carefully followed to exclude bacterial infection and the development of lymphoma.4 The painful parotid swelling and the sicca symptoms were the reasons that led the patient to seek professional guidance.
The oral component of SS can be evaluated in many ways. The subjective feelings and symptoms of xerostomia may have occurred as a result of other diseases (eg, diabetes mellitus; sarcoidoses; infective, respiratory, and renal diseases) and/or lifestyle habits and issues (eg, smoking, drugs, snoring). Therefore, objective tests are needed to verify that xerostomia is, in fact, a result of SS.20 The assessment of salivary gland function (sialometry and/or sialochemistry), salivary gland imaging, and histopatholological examination has been proposed to help confirm the diagnosis of SS.20,21
With regard to the ocular component, the decrease in lacrimal flow can lead to keratoconjuctivitis sicca. Moreover, foreign body sensation, sensations of grittiness, soreness, itching, blurred vision, or light sensitivity may be reported. Some tests are available to objectively quantitate ocular dryness, such as the Schirmer’s test, which was used for this case.1,4
Involvement of other exocrine glands occurs less frequently in SS. However, extraglandular manifestations such as musculoskeletal, pulmonary, gastrointestinal, renal, neurologic, hematologic, and cutaneous signs and symptoms have been associated with SS. Also, lymphoma has been linked with the disease.4 The patient in this case presented only with urticarial vasculitis, without any evidence of other systemic involvement, which agreed with the findings from Ramos-Casals et al,22 who considered the cutaneous manifestations one of the most typical extraglandular features and significant in the prognosis for patients with primary SS.
The most serious complication of SS is the high risk for the occurrence of non-Hodgkin’s lymphoma.4 The case presented shows a persistent parotid gland swelling, xerostomia, xerophthalmia, and the presence of ANA and antibodies to SSA, which are reasons for concern due to the risk for transformation in non-Hodgkin’s lymphoma. Although no evidence of lymphadenopathy or organomegaly was found, the risk for malignant development with time makes a long-term follow-up absolutely mandatory in such patients. In our study, the patient has continued to be followed, and there have been no clinical or laboratory manifestations for the diagnosis of lymphoma.
The goal of the workup for SS is to make a differential diagnosis and to document the key features of SS. Taking a thorough medical history, performing a physical examination, and analyzing images as well as studying the findings of hematologic, immunologic, and histopathologic tests yields comprehensive information to achieve a correct diagnosis. The definitive diagnosis of primary SS was reached using the American-European Consensus Group diagnostic criteria6: the patient had symptoms of xerostomia and xerophthalmia, a positive Schirmer’s test result, positive La autoantibodies and RF, and minor salivary gland biopsy showing a focus score of > 1. Moreover, scialography and ultrasound were performed to assess the parotid gland involvement. All tests were performed and diagnosed by the physician, except the histopathologic examination of minor salivary glands, which was performed by the dental team.
Conclusion
Although the SS diagnosis remains a challenge for clinicians, some oral clinical features may lead to diagnosing the disorder earlier, thus contributing to potentially initiating preventive treatment and avoiding irreversible degenerative glandular changes characterizing advanced-stage disease. Astute clinicians should not underestimate the possible presence or potential development of SS in patients without xerostomia. In patients with these oral features, the key concepts are early recognition and intervention to prevent the secondary complications from hyposalivation. Nevertheless, because of the possible systemic involvement, the patient should be monitored and managed by professionals in several specialties. A coordinated management program among dentists, ophthalmologists, gynecologists, rheumatologists, and primary care physicians is essential for early diagnosis and treatment. In this report, the combination of clinical features, laboratory investigations (serum and immunological examinations), lower lip biopsy, and imaging findings (sialography and ultrasonography) improved the diagnostic accuracy.
About the Authors
Aruanda Fiche, DMD
Private Practice
Belo Horizonte, Brazil
Alynne Vieira Menezes, DDS, PhD
Professor
Department of Oral Radiology
Federal University of Ceará
Fortaleza, Brazil
Claudia Scigliano Valerio, MSD
Doctoral Student
Department of Dentistry
Pontifical Catholic University of Minas Gerais
Belo Horizonte, Brazil
Martinho Campolina Rebello Horta, DDS
Adjunct Professor
Department of Dentistry
Pontifical Catholic University of Minas Gerais
Belo Horizonte, Brazil
Flávio Ricardo Manzi, DDS, PhD
Adjunct Professor
Department of Dentistry
Pontifical Catholic University of Minas Gerais
Belo Horizonte, Brazil
Queries to the author regarding this course may be submitted to authorqueries@aegiscomm.com.
Disclosure
The author had no disclosures to report.
References
1. Carsons S. A review and update of Sjögren’s syndrome: manifestations, diagnosis, and treatment. Am J Manag Care. 2001;7(14 suppl):S433-S443.
2. al-Hashimi I. The management of Sjögren’s syndrome in dental practice. J Am Dent Assoc. 2001;132(10):1409-1417.
3. Shimizu M, Okamura K, Yoshiura K, et al. Sonographic diagnostic criteria for screening Sjögren’s syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102(1):85-93.
4. Mavragani CP, Moutsopoulos NM, Moutsopoulos HM. The management of Sjögren’s syndrome. Nat Clin Pract Rheumatol. 2006;2(5):252-261.
5. Pinto A. Pathobiology of Sjögren syndrome: what do we know? Curr Oral Heal Reports. 2014;1(3):167-172.
6. Shiboski CH, Shiboski SC, Seror R, et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren’s syndrome: a consensus and data-driven methodology involving three international patient cohorts. Arthritis Rheumatol. 2016;69(1):35-45.
7. Rasmussen A, Ice JA, Li H, et al. Comparison of the American-European Consensus Group Sjogren’s syndrome classification criteria to newly proposed American College of Rheumatology criteria in a large, carefully characterised sicca cohort. Ann Rheum Dis. 2014;73(1):31-38.
8. Steinfeld SD, Demols P, Appelboom T. Infliximab in primary Sjögren’s syndrome: one-year followup. Arthritis Rheum. 2002;46(12):3301-3303.
9. Johnson EO, Vlachoyiannopoulos PG, Skopouli FN, et al. Hypofunction of the stress axis in Sjögren’s syndrome. J Rheumatol. 1998;25(8):1508-1514.
10. Kassan SS, Moutsopoulos HM. Clinical manifestations and early diagnosis of Sjögren syndrome. Arch Intern Med. 2004;164(12):1275-1284.
11. Porter SR, Scully C, Hegarty AM. An update of the etiology and management of xerostomia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97(1):28-46.
12. Boutsi EA, Paikos S, Dafni UG, et al. Dental and periodontal status of Sjögren’s syndrome. J Clin Periodontol. 2000;27(4):231-235.
13. Najera MP, Al-Hashimi I, Plemons JM, et al. Prevalence of periodontal disease in patients with Sjögren’s syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83(4):453-457.
14. Scardina GA, Ruggieri A, Messina P. Periodontal disease and sjogren syndrome: a possible correlation? Angiology. 2010;61(3):289-293.
15. Zero DT, Brennan MT, Daniels TE, et al. Clinical practice guidelines for oral management of Sjögren disease: Dental caries prevention. J Am Dent Assoc. 2016;147(4):295-305.
16. Soto-Rojas AE, Kraus A. The oral side of Sjögren syndrome. Diagnosis and treatment. A review. Arch Med Res. 2002;33(2):95-106.
17. Cartee DL, Maker S, Dalonges D, Manski MC. Sjögren’s syndrome: oral manifestations and treatment, a dental perspective. J Dent Hyg. 2015;89(6):365-371.
18. Pinto A. Management of xerostomia and other complications of Sjögren’s syndrome. Oral Maxillofac Surg Clin North Am. 2014;26(1):63-73.
19. Kulkarni K. Unusual presentation of Sjögren syndrome. South Med J. 2005;98(12):1210-1211.
20. El Miedany YM, Ahmed I, Mourad HG, et al. Quantitative ultrasonography and magnetic resonance imaging of the parotid gland: can they replace the histopathologic studies in patients with Sjogren’s syndrome? Joint Bone Spine. 2004;71(1):29-38.
21. Aoun G, Nasseh I, Berberi A. Evaluation of the oral component of Sjögren’s syndrome: An overview. J Int Soc Prev Community Dent. 2016;6(4):278-284.
22. Ramos-Casals M, Tzioufas AG, Font J. Primary Sjögren’s syndrome: new clinical and therapeutic concepts. Ann Rheum Dis. 2005;64(3):347-354.