You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
A total of 75% of the US population has some form of malocclusion, according to Proffit et al.1 With the aging population’s longer lifespans and desire for better quality of life, the demand for orthodontic services has increased. In general, the most common concern for adult patients considering orthodontia is treatment time. Although several novel modalities have been reported to accelerate orthodontic tooth movement, including low-level laser therapy,2 pulsed electromagnetic fields,3 electrical currents,4 distraction osteogenesis,5 and mechanical vibration,6 current evidence suggests only corticotomy-accelerated orthodontics (CAO) shows a clear benefit.7
CAO has been popularized under the name Wilckodontics®. It is also referred to as periodontally accelerated osteogenic orthodontics (PAOO), Accelerated Osteogenic Orthodontics™ (AOO™), accelerated orthodontics (AO), selective alveolar decortication (SAD), surgically facilitated orthodontic therapy (SFOT), and corticotomy-facilitated orthodontics (CFO).8-11 The only difference is that SFOT, AOO, and PAOO involve bone grafting in addition to corticotomy. In general with SFOT, corticotomy and bone grafting may be performed only in the direction of tooth movements rather than on both buccal and lingual/palatal aspects.
Before explaining the CAO procedure, three surgical terminologies should be described: osteotomy, corticotomy, and PAOO.
Osteotomy is a surgical cut made through both the cortical and medullary components of the bone so as to free a bone segment and allow distraction histogenesis to occur. This is often referred to as dentoalveolar distraction osteogenesis. Corticotomy is a surgical procedure in which the cortical bone is injured with some injury extending into the medullary bone. The corticotomy may be shallow or deep. The intention is to perforate or mechanically alter the cortical bone to create a purposeful injury for a known therapeutic benefit that is generally engineered orthodontically. This occurs around the tooth but without damaging it. It is also accompanied by the use of dentoalveolar decortication of the alveolus. PAOO is a technique that combines selective alveolar corticotomy, particulate bone grafting, and the application of orthodontic force.10
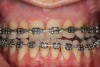
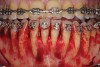
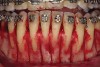
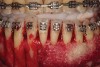
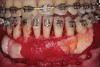
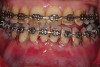
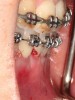
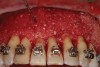
As such, CAO is a surgical procedure involving selectively decorticating (perforating/removing only the cortical layer of) the alveolar bone around the roots of teeth intended to be moved orthodontically in an attempt to accelerate the tooth movement. Figure 1 through Figure 10 illustrates a case treated with corticotomy cuts and dentoalveolar decortication to accelerate orthodontic treatment. Although the primary surgical technique has undergone few changes since it was first described by Cunningham in 1898 and reported by Bichlmayr in 1931,12 the basics of understanding wound healing have become more clearly elucidated in recent years. In 1959, Kole postulated when the thickness and continuity of the dense dentoalveolar bone was disrupted, the teeth could move more freely and quicker. Hence, he made so-called “bone block” cuts in the interdental bone both buccally and lingually and joined them with a subapical osteotomy going through the entire thickness of the alveolus. The bone blocks were described as being connected only by bone marrow, allowing easy movement of the whole segment when orthodontic forces were applied. This approach became known as the bone block theory.13 Figure 11 is a flowchart demonstrating the evolution of the CAO technique since 1931.
The Wilckos’ Concepts
Since Bichlmayr reported his approach, many have tried to modify the surgical technique. Many consider the work by William and Thomas Wilcko to have made the most significant contributions to this discipline in modern-day periodontics and orthodontics.10
Based on Frost’s work14 with bone healing and his descriptions of the regional acceleratory phenomenon (RAP) during fracture healing, the Wilckos proposed several critical concepts explaining the mechanism by which teeth move faster after corticotomy.10 They found that the alveolar bone following a corticotomy loses structural integrity as a result of a transient demineralization.15 They described the healing as a soft collagenous bone matrix that remained within the dentoalveolar segment and facilitated the orthodontic transportation of the teeth. In addition, this matrix was the source of remineralization when the tooth was retained in its final position. Thus, the healing pattern for accelerated tooth movement caused by the corticotomy and dentoalveolar decortication surgical insult is coupled with the demineralization–remineralization process. This has been well confirmed in a rat model.16 This phenomenon of a “bone matrix transportation” was described because the teeth do not actually move through the dentoalveolar bone segment as with conventional pressure-tension orthodontia, but instead they move with the dentoalveolar bone segment that is being displaced in total during its demineralized state.17 Figure 12 demonstrates particulate bone grafting after corticotomy and dentoalveolar decortication as a part of the SFOT approach. Figure 13 and Figure 14 show cross-sectional cone-beam computed tomography images of tooth No. 6 before and after SFOT. When seen together, the images demonstrate improved incisor angle position and axial inclination of the cuspid as well as a significantly improved dentoalveolar bone volume post-treatment.
Similar observations were found incidentally in patients receiving treatment with orthognathic surgery. Orthodontists occasionally reported patients’ teeth tended to move faster after orthognathic surgery, which can be explained only by the RAP effect. This led to a relatively new trend of a “surgery first” approach, which means orthognathic surgery is performed before orthodontic treatment.18 Research19 has found the surgery-first approach led to accelerated tooth movement within the first 3 months postsurgical, resulting in superior satisfaction for both the patient and orthodontist. However, the main limitation of CAO is involvement of the surgical procedure, which sometimes prohibits patients from accepting this treatment or even orthodontists from recommending it. Other limitations will be highlighted below.
Description of the Technique
The Role of the Surgeon
The primary role of the surgeon is to surgically engineer the case (addressing dentoalveolar bone and periodontal soft tissues) to meet or exceed the expectations of the orthodontist, who is setting up the treatment from esthetic, occlusal, and airway perspectives. The role of the surgeon is also to contribute to the engineering of the orthodontic setup and plan and then execute periodontal and dentoalveolar surgery so that the objectives of orthodontic therapy are realized. The surgeon must understand the cephalometric diagnostics and craniofacial analysis of the patient.
Flap Design
The flap design is a full-thickness mucoperiosteal flap with a periosteal-releasing incision at the base to provide mobility so as to attain primary closure over the bone graft.20 In certain instances, the interdental papilla must be preserved to gain a better esthetic outcome.20 This technique was first described by Velvart,21 who labeled this as a papilla base flap. It is often preferable to avoid vertical incisions to limit vascular embarrassment.22 The Takei papilla preservation design may also be chosen.23 Figure 15 shows a papilla-base incision/preferred flap design and the Takei papilla preservation technique at teeth Nos. 9 and 10. If an envelope design is chosen, the clinician is advised to extend the flap two to three teeth beyond the area in which the corticotomy cuts are to be performed.20
Corticotomy
The instrumentation involved in corticotomy surgery can involve the use of a round bur mounted on a surgical handpiece,10 a piezoelectric tip,24 microsaws,25 reinforced surgical blades that are introduced through the interdental bone by a mallet,26 or even erbium-doped yttrium aluminium garnet (Er:YAG) lasers.27 Vertical grooves are extended from a point 2 mm to 3 mm below the crest of the interdental bone to an apical point within the dentoalveolar complex no less than 5 mm beyond the apexes of the roots.28 The vertical corticotomy cuts are connected with an apically displaced and connecting corticotomy no less than 5 mm from the tooth apex. The decision to perform corticotomy on the buccal and lingual bone plates generally depends on how much of the RAP effect is required around the dentoalveolar segments and how much tooth movement is needed. If the alveolar bone is of sufficient thickness, solitary perforations may be placed in the alveolar bone over the radicular surface. However, if this bone is estimated to be less than 1 mm to 2 mm in thickness, these perforations are omitted to ensure no damage occurs to the radicular surface.20 Because this technique is mediated by the periodontal ligament (PDL), though, the injury must be near the PDL so that the RAP effect occurs in this region. In the presence of thick dentoalveolar bone, mild corticotomy surgery may not produce enough injury and associated RAP to appreciably expedite tooth movement.29 This is a PDL-mediated process. Though the surgery occurs around the dentoalveolar compartment, the benefits related to tooth movement are a result of the coupled demineralization–remineralization process affecting the PDL and alveolar bone proper.
The current trend toward making the surgery more conservative is not fully consistent with the RAP concept. Though any injury of bone will induce a RAP effect, it seems to be directly proportional with the amount of injury.29,30 It has been stressed that the alveolar bone over the root prominences should be thinned to approximately 1.5 mm in the direction of tooth movement with an intent to permit the collagenous matrix to be transferred with the roots.15 The degree of tissue injury is the key behind the accelerated tooth movement, not the design of the decortication.31
The Need for Bone Augmentation
The decision for whether to place a bone graft should be influenced by the direction and amount of tooth movement needed, pretreatment thickness of the alveolar bone, and the age of the patient.17 The most important reason for grafting is to be able to change the alveolar bone shape and volume as needed so the movement will not be restricted by the available bony architecture.17,32 In addition, alveolar bone grafting has been shown to successfully correct bony dehiscences existing at the time of surgery.10,17,24,31 If the bone thickness around the roots is more than 1.5 mm, then grafting may not be necessary.31 Mandelaris and colleagues33 have proposed a pretreatment classification of dentoalveolar bone phenotypes to help clinicians understand the risk associated with tooth movement and help in determining whether SFOT with or without alveolar augmentation is indicated. One study has shown CAO without bone grafting resulted in complete healing in adolescents without any net tissue loss.34 In contrast, it demonstrated incomplete healing accompanied by a small amount of tissue loss in adults. In addition, a controlled study showed bone density decreased after treatment and was back to normal after 6 months of retention in the control group (CAO without bone graft). Bone density increased by 26% in the test group (CAO with bone graft).35
Unexpectedly, using barrier membranes has never been suggested in any description of the technique. However, in the case of fenestration and/or dehiscence around roots, if no alveolar augmentation is being performed in the direction of tooth movement where the preexisting dentoalveolar bone is thin, further bone loss may occur. This highlights the need for some consideration of using a barrier membrane to prevent such adverse sequelaes.36 The Wilckos and colleagues suggested limiting the use of the barrier membrane only to the areas receiving dental implants after debracketing.31 Nonetheless, a road map based on preoperative CBCT and clinical photos also may be used to better plan the surgery and to determine whether the grafting is needed.24 Presurgical planning is paramount for choosing the most appropriate flap design and deciding whether a bone/soft-tissue grafting is required.
Operative Recommendations
Depending on the scope of treatment, surgery may take 1 to 3 hours per arch. Intravenous conscious sedation or general anesthesia is often recommended to ensure airway protection during surgery. Because of the possible postsurgical edema associated from flap reflection, steroids may be prescribed to patients pre- and postsurgery.20 Antibiotics are used preoperatively and prescribed for 7 to 10 days postoperatively, as well as acetaminophen with or without narcotics for pain management. Nonsteroidal anti-inflammatory drugs (NSAIDs) may be avoided so that inflammation-induced demineralization can be maximized. Figure 16 demonstrates a CAO case with bone and accelular dermal matrix grafting to manage a patient with mild maxillary transverse deficiency as well as a dental and skeletal class III malocclusion with significant dentoalveolar deficiencies. The bone augmentation allowed for tooth movement to occur within an expanded bone envelope that was previously deficient. This enhanced the dentoalveolar bone phenotype and allowed an increase in the orthodontic walls in which tooth movement could be more safely produced. Finally, expanding the bone envelope allowed controlled tipping and uprighting of the root to occur, which would have otherwise displaced the root further outside the known orthodontic tooth limits37 and likely caused iatrogenic sequelaes secondary to tooth movement. The bone (and, in this case, soft-tissue) augmentation allowed more favorable root positioning to occur and avoided the need for orthodontic camouflaging. Better axial inclination and incisor angle relationship were produced for a mild skeletal malocclusion, which also overcame the need for orthognathic surgery to enable coupling of the anterior teeth.
Planning the Procedure With the Surgeon
The role of the orthodontist is to engineer the case for optimal dentofacial, occlusion, and airway outcomes. For instance, the orthodontist’s role can fall into determining where dentoalveolar bone should be demineralized to facilitate tooth movement and how or whether anchorage (via temporary anchorage devices [TADs], anchor plates, or unactivated teeth) should be used to optimize the post-CAO mechanics of the case. The orthodontist must be aware of the preexisting dentoalveolar bone phenotype of the patient and ensure that the periodontium is not compromised as a result of tooth movement (ie, tooth movement outside the orthodontic walls). From the orthodontic setup and treatment planning with the surgeon, an interdisciplinary plan is developed. Careful coordination between the surgeon and orthodontist is required for successful outcomes. If prosthetic dentistry is to be incorporated into the patient’s treatment plan, the restorative dentist and/or prosthodontist also should be involved in the planning so that tooth position, anterior-protected articulation, and space appropriation are optimized for the patient. Depending on the case and the planning from the orthodontist, aligner trays may be considered on a case-by-case basis. The popularity of invisible aligner trays in the adult population and the trays’ enhanced ability to maintain plaque control make this an attractive consideration if the orthodontist can achieve the same results compared with conventional appliance therapy. The biology of tooth movement does not change depending on the type of appliance used unless an insult such as corticotomy surgery occurs to disrupt the mechanism of action.
Starting the Tooth Movement Within the First Week of Surgery
It is generally recommended to have orthodontic appliances on the teeth before surgery.31 Meaningful orthodontic tooth movement should begin 7 to 10 days postsurgery, during which the RAP effect should be present within the dentoalveolar complex. In one report, the largest decrease in bone density was recorded immediately after the surgery and for 3 months afterward.38
Making Use of the ‘Open Window’
Frost30 reported that RAP peaked at 1 to 2 months after injury and the effect started to fade gradually for up to 2 years. Sebaoun and colleagues39 observed in a rat model 3 weeks after surgery, the bone metabolism increased and returned to normal by the eleventh week postoperatively. Another report found that the amount of tooth movement achieved within the first 2 months was significantly higher than in the following 2 months.40 Therefore, in the first 3 to 4 months following the corticotomy surgery, most of the significant orthodontic treatment should be attempted to be completed.41-43 Therefore, adjustments should be performed every 1 to 2 weeks as teeth are expected to achieve movement faster than usual during this period.31,44
Benefits
Several benefits have been associated with the use of CAO. These include, but are not limited to, the following: shortened orthodontic treatment time, reduced relapse rate after orthodontic treatment, and potential increases in supporting alveolar bone thickness.
More recently, concepts of airway improvements are being investigated, which may prove to have systemic health benefits.45 It has been shown that patients with larger oral-cavity volumes tend to trend away from sleep-disordered breathing compared to those with smaller oral-cavity volumes. Given the emerging epidemic of sleep-disordered breathing in the United States,46 clinicians should avoid the use of extraction therapy and retractive orthodontics when possible. CAO and alveolar augmentation may help increase the maxillary and/or mandibular bone-volume availability to allow for more arch length and therefore reduce the need for bicuspid extraction and retractive orthodontia. The consideration of airway dimensions by using SFOT to avoid retractive orthodontia—when possible—may help trend susceptible patients away from sleep-disordered breathing conditions, as well as actually reduce the risk for sleep apnea conditions and their compounded effects on the chronic diseases of aging.47 From a dental perspective, improved oral-cavity volume generally leads to a more attractive smile, better occlusion, and improved dentofacial esthetics.48,49
Indications
One indication is for accelerating orthodontic treatment. Shortening the duration of orthodontic treatment in extraction and nonextraction treatment of crowding via CAO is well established in the literature.10,17,20,34,41,51 CAO has been found to have a much lower rate of loss of anchorage.52 Recently, in a case treated with clear aligners in combination with corticotomy, the orthodontic treatment was completed in just 2 months.53
Another indication is for treating borderline orthognathic surgery cases. These cases were successfully treated by the use of CAO because the dentoalveolar bone volume could be enhanced, allowing for teeth to be moved beyond typical physiologic limits. CAO and alveolar augmentation allow the orthodontic walls to be expanded,54 whereby mild skeletal discrepancies can be treated with less-invasive surgery.38,51,55
CAO has also been used in the treatment of severe anterior open bites in conjunction with skeletal anchorage. It has been found that the combination of a skeletal anchorage system with corticotomy resulted in a 3-mm intrusion of maxillary molars within 2 months, with no reported root resorption or problems with patient compliance.56 Others have reported that with CAO, a 4-mm intrusion of molars can be completed in about 2.5 months.38
CAO has been shown to significantly speed the treatment time (two to three times) of canine retraction when compared to the sites without CAO.43
CAO can also be utilized for minimizing postorthodontic relapse. Relapse is a major challenge for traditional orthodontic treatment. It has been shown only 30% of orthodontics patients had no signs of relapse.57 Some studies have indicated with CAO use, the incidence of orthodontic relapse is minimized.17 A reduction in the incidence of postorthodontic relapse following CAO-related treatment may occur because such therapy induces a higher periodontium turnover, which may lead to loss of tissue memory,32 and/or because additional bone grafting placed during CAO promotes new bone formation and stabilizes teeth in the new position,34 perhaps as a result of remineralization that occurs after treatment.17
CAO can also be used in many other potential clinical situations, such as extrusion of ankylosed teeth55 and traction of impacted third molars.58 However, these applications require further validation.
Contraindications
CAO is contraindicated in patients with any of the following: signs of active periodontal disease, inadequately treated endodontic problems, prolonged use of corticosteroids, and use of medications that slow bone metabolism, such as bisphosphonate and perhaps NSAIDs.31
Potential Complications
Below are several complications or adverse effects that have been associated with CAO treatment.
Possible loss of tooth vitality: Most studies showed minimal to no changes of tooth vitality following corticotomy procedures,28,41,55,59 yet there is not enough evidence to support this statement. Further research in this area is needed.
Jeopardizing the surrounding periodontium: The use of CAO has been thought to jeopardize the overall periodontal condition because of flap opening and cortical bone reduction.60 So far, the literature has reported no adverse effect on CAO use toward the surrounding periodontium.43,61 Furthermore, it has been shown that CAO actually reduced the mean pocket depth by about 0.2 mm to 1.5 mm when compared to sites that did not receive treatment with CAO.35 In addition, some have reported that CAO use increases the dentoalveolar bone width10,62 and the zone of keratinized tissue height.63
Root resorption: The use of CAO has not been shown to increase the risk for root resorption17 when compared with the expected root resorption during conventional pressure-tension–mediated orthodontic tooth movement.64 Conversely, a positive correlation has been shown between increased root resorption and duration of the applied force.65 Hence, the reduction of treatment time via CAO use may actually reduce the risk for root resorption because CAO bypasses the lag phase of orthodontic therapy, which is responsible for hyalinization of the PDL.16 This is further confirmed by an animal study finding, which reported no incidence of root resorption associated with CAO treatment.66
Postoperative discomfort: To the best of our knowledge, the only controlled study assessing patient-reported outcomes after CAO was comparing piezoelectric and rotary instruments for the corticotomy.67 Results from this study showed occasional pain in both groups and that was noted in only the first week postsurgery.
Current Evidence for CAO Treatment
The Time Saved by CAO
Research has clearly demonstrated that the use of CAO can shorten the overall orthodontic treatment. It has been reported that tooth movement was achieved three to four times faster than with the control group.40 For example, in a case with severe bimaxillary protrusion, complete retraction of anterior teeth combined with CAO was completed in less than 3.5 months.68 For orthodontic treatment in the nonextraction cases, the treatment could be completed in 6 months, instead of 18 months using a conventional orthodontic approach.69 Nonetheless, one study showed “less than expected” speed of tooth movement after the use of CAO;26 however, this may have occurred as a result of not following the recommended surgical protocol.44
Minimization of Orthodontic Post-treatment Relapse
Makki and colleagues70 have shown that the incidence of orthodontic relapse as evaluated by the mandibular irregularity index is significantly less over 10 years compared to traditional orthodontic treatment. Other than this study, all available literature comprises a few case reports.71 It should be noted that several factors contribute to the relapse process. Hence, it is not easy to tie this to one factor solely. It is, therefore, advised not to promise patients that CAO can minimize post-treatment relapse until more convincing evidence is available.
Increased Bone Width After Surgery
The increase in bony width after CAO is a common finding in cases in which a bone graft was added.17,31 Some have shown that additional bone grafting during the use of CAO promotes complete bone formation.31 In contrast, the key question is this: Are any complications noted when performing CAO without bone grafting? More evidence in this area is needed. Nonetheless, bone grafting is often beneficial because of the fragility of the facial bone. In a study of nearly 500 patients for whom cone-bean computed tomography imaging was used to evaluate facial-bone thickness from the maxillary first premolar to the contralateral maxillary first premolar, Braut and colleagues72 showed that, on average, 90% of patients studied had less than 1 mm of facial-bone thickness. Facial bone augmentation is, therefore, often an advantage. Also, bone formation depends on adhering to surgical and biologic principles such as PASS73 for optimal healing and outcome predictability. (PASS principles refer to primary wound closure, angiogenesis, space creation or maintenance, and stability of the blood clot and implant.) In most cases, bone-graft placement will result in increasing bone width.
Flap vs Flapless Surgery
Attempts to perform CAO without a flap have been proposed.26 One technique involved using a reinforced scalpel introduced by a mallet to penetrate the gingiva and cortical bone without raising a flap buccally and lingually.26 Later, the Piezocision™ technique was introduced, in which interproximal incisions are made by blade, followed by piezoelectric corticotomy through those incisions.24 Sites that needed either bone or soft-tissue graft were treated using a tunneling approach without raising the flap. Recently, Cassetta and colleagues74 proposed an innovative, minimally invasive flapless procedure combining piezoelectric surgical cortical incisions with the use of a 3-dimensional–printed computer-aided design and computer-aided manufacturing (CAD/CAM) surgical guide. This technique overcomes the obvious drawbacks of other flapless procedures such as being surgically blind, which might cause root injury.24 Nonetheless, the suggested approach requires more data to support its clinical usage. In this context, the orthodontic walls are not enhanced as predictably because access and visualization are not ensured, and the position and stability of the bone augmentation can be jeopardized.
Combination Approaches
Researchers have tried to combine the use of CAO with other treatment procedures that have previously proved to decrease the treatment time so as to maximize the speed of tooth movement. It has been reported that CAO use conjoined with the use of miniscrews can successfully retract maxillary canines into the sites of extracted premolars.43 This study showed this combination approach resulted in two-times-faster tooth movement than in the groups without the use of miniscrews in the first 2 months. However, others have failed to prove the benefits of using them.40 More studies are needed to verify the advantage of using additional miniscrews.
It has also been shown that the use of low-level laser therapy (LLLT) could facilitate tooth movement.75 However, a study by Han and colleagues76 failed to show the application of LLLT could enhance the outcome of CAO treatment. Research is needed to determine the benefit of using LLLT during CAO treatment.
Conclusions
The use of CAO is a safe and effective technique to accelerate orthodontic tooth movement (an average of three times faster compared to the traditional approach). However, the evidence supporting other claimed benefits requires further investigation. More studies are needed to either confirm or reject some of these claims. In the meantime, CAO represents an exciting and emerging field that can expand the scope of orthodontic therapy and has clear benefits on the periodontium when used as a part of comprehensive interdisciplinary dentofacial therapy.
About the Authors
Nouf Zimmo, DDS
Postgraduate Student, Graduate Periodontics, Department of Periodontics and Oral Medicine, University of Michigan School of Dentistry, Ann Arbor, Michigan
Muhammad H.A. Saleh, BDS
Graduate Periodontics, Department of Periodontics and Oral Medicine, University of Michigan School of Dentistry, Ann Arbor, Michigan
George A. Mandelaris, DDS, MS
Adjunct Clinical Assistant Professor, Department of Periodontics, University of Illinois at Chicago College of Dentistry, Chicago, Illinois; Private Practice, Oakbrook Terrace, Lincoln Park and Park Ridge, Illinois
Hsun-Liang Chan, DDS, MS
Clinical Assistant Professor, Department of Periodontics and Oral Medicine, University of Michigan School of Dentistry, Ann Arbor, Michigan
Hom-Lay Wang, DDS, MSD, PhD
Professor and Director, Graduate Periodontics, Department of Periodontics and Oral Medicine, University of Michigan School of Dentistry, Ann Arbor, Michigan
Queries to the authors regarding this course may be submitted to authorqueries@aegiscomm.com.
REFERENCES
1. Proffit WR, Fields HW Jr, Moray LJ. Prevalence of malocclusion and orthodontic treatment need in the United States: estimates from the NHANES III survey. Int J Adult Orthodon Orthognath Surg. 1998;13(2):97-106.
2. Cruz DR, Kohara EK, Ribeiro MS, Wetter NU. Effects of low-intensity laser therapy on the orthodontic movement velocity of human teeth: a preliminary study. Lasers Surg Med. 2004;35(2):117-120.
3. Showkatbakhsh R, Jamilian A, Showkatbakhsh M. The effect of pulsed electromagnetic fields on the acceleration of tooth movement. World J Orthod. 2010;11(4):e52-e56.
4. Kim DH, Park YG, Kang SG. The effects of electrical current from a micro-electrical device on tooth movement. Korean J Orthod. 2008;38(5):337-346.
5. Liou EJ, Huang CS. Rapid canine retraction through distraction of the periodontal ligament. Am J Orthod Dentofacial Orthop. 1998;114(4):372-382.
6. Nishimura M, Chiba M, Ohashi T, et al. Periodontal tissue activation by vibration: intermittent stimulation by resonance vibration accelerates experimental tooth movement in rats. Am J Orthod Dentofacial Orthop. 2008;133(4):572-583.
7. Long H, Pyakurel U, Wang Y, et al. Interventions for accelerating orthodontic tooth movement: a systematic review. Angle Orthod. 2013;83(1):164-171.
8. Roblee RD, Bolding SL, Landers JM. Surgically facilitated orthodontic therapy: a new tool for optimal interdisciplinary results. Compend Contin Educ Dent. 2009;30(5):264-275; quiz 276.
9. Hoogeveen EJ, Jansma J, Ren Y. Surgically facilitated orthodontic treatment: a systematic review. Am J Orthod Dentofacial Orthop. 2014;145(4 suppl):S51-S64.
10. Wilcko WM, Wilcko T, Bouquot JE, Ferguson DJ. Rapid orthodontics with alveolar reshaping: two case reports of decrowding. Int J Periodontics Restorative Dent. 2001;21(1):9-19.
11. Liem AM, Hoogeveen EJ, Jansma J, Ren Y. Surgically facilitated experimental movement of teeth: systematic review. Br J Oral Maxillofac Surg. 2015;53(6):491-506.
12. Bichlmayr AChirurgische Kieferothopaedie und das Verhalten des Knochens und der Wurzelspitzennachderselben. Dtsch Zahnaerztl Wschr. 1931;34:835-842.
13. Kole H. Surgical operations on the alveolar ridge to correct occlusal abnormalities. Oral Surg Oral Med Oral Pathol. 1959;12(5):515-529.
14. Frost HM. The regional acceleratory phenomenon: a review. Henry Ford Hosp Med J. 1983;31(1):3-9.
15. Wilcko WM, Ferguson DJ, Bouquot JE, Wilcko MT. Rapid orthodontic decrowding with alveolar augmentation: case report. World J Orthod. 2003;4(3):197-205.
16. Baloul SS, Gerstenfeld LC, Morgan EF, Carvalho RS, Van Dyke TE, Kantarci A. Mechanism of action and morphologic changes in the alveolar bone in response to selective alveolar decortication-facilitated tooth movement. Am J Orthod Dentofacial Orthop. 2011;139(4 suppl):S83-S101.
17. Wilcko MT, Wilcko WM, Bissada NF. An evidence-based analysis of periodontally accelerated orthodontic and osteogenic techniques: a synthesis of scientific perspectives. Semin Orthod. 2008;14:305-316.
18. Liou EJ, Chen PH, Wang YC, Yu CC, Huang CS, Chen YR. Surgery-first accelerated orthognathic surgery: orthodontic guidelines and setup for model surgery. J Oral Maxillofac Surg. 2011;69(3):771-780.
19. Hernandez-Alfaro F, Guijarro-Martinez R, Peiro-Guijarro MA. Surgery first in orthognathic surgery: what have we learned? A comprehensive workflow based on 45 consecutive cases. J Oral Maxillofac Surg. 2014;72(2):376-390.
20. Murphy KG, Wilcko MT, Wilcko WM, Ferguson DJ. Periodontal accelerated osteogenic orthodontics: a description of the surgical technique. J Oral Maxillofac Surg. 2009;67(10):2160-2166.
21. Velvart P. Papilla base incision: a new approach to recession-free healing of the interdental papilla after endodontic surgery. Int Endod J. 2002;35(5):453-460.
22. Kleinheinz J, Buchter A, Kruse-Losler B, et al. Incision design in implant dentistry based on vascularization of the mucosa. Clin Oral Implants Res. 2005;16(5):518-523.
23. Takei HH, Han TJ, Carranza FA Jr, et al. Flap technique for periodontal bone implants. Papilla preservation technique. J Periodontol. 1985;56(4):204-210.
24. Dibart S, Sebaoun JD, Surmenian J. Piezocision: a minimally invasive, periodontally accelerated orthodontic tooth movement procedure. Compend Contin Educ Dent. 2009;30(6):342-344, 346, 348-350.
25. Duker J. Experimental animal research into segmental alveolar movement after corticotomy. J Maxillofac Surg. 1975;3(2):81-84.
26. Kim SH, Kook YA, Jeong DM, Lee W, Chung KR, Nelson G. Clinical application of accelerated osteogenic orthodontics and partially osseointegrated mini-implants for minor tooth movement. Am J Orthod Dentofacial Orthop. 2009;136(3):431-439.
27. Salman LH, Ali FA. Acceleration of canine movement by laser assisted flapless corticotomy [an innovative approach in clinical orthodontics]. Journal of Baghdad College of Dentistry. 2014;26(3):133-137.
28. Ozturk M, Doruk C, Ozec I, et al. Pulpal blood flow: effects of corticotomy and midline osteotomy in surgically assisted rapid palatal expansion. J Craniomaxillofac Surg. 2003;31(2):97-100.
29. McBride MD, Campbell PM, Opperman LA, et al. How does the amount of surgical insult affect bone around moving teeth? Am J Orthod Dentofacial Orthop. 2014;145(4 suppl):S92-S99.
30. Frost HM. The biology of fracture healing. An overview for clinicians. Part I. Clin Orthop Relat Res. 1989;(248):283-293.
31. Wilcko MT, Wilcko WM, Pulver JJ, et al. Accelerated osteogenic orthodontics technique: a 1-stage surgically facilitated rapid orthodontic technique with alveolar augmentation. J Oral Maxillofac Surg. 2009;67(10):2149-2159.
32. Ferguson DJ, Wilcko WM, Wilcko MT. Selective alveolar decortication for rapid surgical-orthodontic correction of skeletal malocclusion. In: Bell WH, Guerrero CA, eds. Distraction Osteogenesis of the Facial Skeleton. Hamilton, ON: BC Decker; 2007:199-203.
33. Mandelaris GA, Vence BS, Rosenfeld AL, Forbes DP. A classification system for crestal and radicular dentoalveolar bone phenotypes. Int J Periodontics Restorative Dent. 2013;33(3):289-296.
34. Wilcko MT, Wilcko WM, Marquez MG, Ferguson DJ. The contribution of periodontics to orthodontic therapy. In: Dibart S, ed. Practical Advanced Periodontal Surgery. Wiley Blackwell, Ames. 2007:23-50.
35. Shoreibah EA, Ibrahim SA, Attia MS, Diab MM. Clinical and radiographic evaluation of bone grafting in corticotomy-facilitated orthodontics in adults. J Int Acad Periodontol. 2012;14(4):105-113.
36. Fu JH, Oh TJ, Benavides E, et al. A randomized clinical trial evaluating the efficacy of the sandwich bone augmentation technique in increasing buccal bone thickness during implant placement surgery: I. Clinical and radiographic parameters. Clin Oral Implants Res. 2014;25(4):458-467.
37. Handelman CS. The anterior alveolus: its importance in limiting orthodontic treatment and its influence on the occurrence of iatrogenic sequelae. Angle Orthod. 1996;66(4):246.
38. Oliveira DD, de Oliveira BF, de Araujo Brito HH, et al. Selective alveolar corticotomy to intrude overerupted molars. Am J Orthod Dentofacial Orthop. 2008;133(6):902-908.
39. Sebaoun JD, Kantarci A, Turner JW, et al. Modeling of trabecular bone and lamina dura following selective alveolar decortication in rats. J Periodontol. 2008;79(9):1679-1688.
40. Al-Naoum F, Hajeer MY, Al-Jundi A. Does alveolar corticotomy accelerate orthodontic tooth movement when retracting upper canines? A split-mouth design randomized controlled trial. J Oral Maxillofac Surg. 2014;72(10):1880-1889.
41. Suya H. Corticotomy in orthodontics. In: Hosl E, Baldauf A, eds. Mechanical and Biological Basics in Orthodontic Therapy. Heidelberg, Germany: Huthig; 1991:207-226.
42. Iino S, Sakoda S, Ito G, et al. Acceleration of orthodontic tooth movement by alveolar corticotomy in the dog. Am J Orthod Dentofacial Orthop. 2007;131(4):448.e1-448.e8
43. Aboul-Ela SM, El-Beialy AR, El-Sayed KM, et al. Miniscrew implant-supported maxillary canine retraction with and without corticotomy-facilitated orthodontics. Am J Orthod Dentofacial Orthop. 2011;139(2):252-259.
44. Murphy NC. Accelerated osteogenic orthodontics. Am J Orthod Dentofacial Orthop. 2010;137(1):2; author reply 2-3.
45. Iida-Kondo C, Yoshino N, Kurabayashi T, et al. Comparison of tongue volume/oral cavity volume ratio between obstructive sleep apnea syndrome patients and normal adults using magnetic resonance imaging. J Med Dent Sci. 2006;53(2):119-126.
46. Ram S, Seirawan H, Kumar SK, Clark GT. Prevalence and impact of sleep disorders and sleep habits in the United States. Sleep Breath. 2010;14(1):63-70.
47. Iida-Kondo C, Yoshino N, Kurabayashi T, et al. Comparison of tongue volume/oral cavity volume ratio between obstructive sleep apnea syndrome patients and normal adults using magnetic resonance imagine. J Med Dent Sci. 2006;53(2):119-126.
48. Ackerman JL, Proffit WR, Sarver DM, et al. Pitch, roll, and yaw: describing the spatial orientation of dentofacial traits. Am J Orthod Dentofacial Orthop. 2007;131(3):305-310.
49. Proffit WR. Masters of esthetic dentistry: the soft tissue paradigm in orthodontic diagnosis and treatment planning: a new view for a new century. J Esthet Dent. 2000;12.1:46-49.
50. Andrews LF, Andrews WA. The six elements of orofacial harmony. Andrews J Orthod Orafac Harmony. 2000;1:36-38.
51. Vercellotti T, Podesta A. Orthodontic microsurgery: a new surgically guided technique for dental movement. Int J Periodontics Restorative Dent. 2007;27(4):325-331.
52. Mostafa YA, Mohamed Salah Fayed M, Mehanni S, et al. Comparison of corticotomy-facilitated vs standard tooth-movement techniques in dogs with miniscrews as anchor units. Am J Orthod Dentofacial Orthop. 2009;136(4):570-577.
53. Cassetta M, Altieri F, Barbato E. The combined use of corticotomy and clear aligners: A case report. Angle Orthod. 2016;86(5):862-870.
54. Handelman C. Palatal expansion in adults: the nonsurgical approach. Am J Orthod Dentofacial Orthop. 2011;140(4):462, 464, 466 passim.
55. Bertossi D, Vercellotti T, Podesta A, Nocini PF. Orthodontic microsurgery for rapid dental repositioning in dental malpositions. J Oral Maxillofac Surg. 2011;69(3):747-753.
56. Moon CH, Wee JU, Lee HS. Intrusion of overerupted molars by corticotomy and orthodontic skeletal anchorage. Angle Orthod. 2007;77(6):1119-1125.
57. Little RM. Stability and relapse of dental arch alignment. Br J Orthod. 1990;17(3):235-241.
58. Ma Z, Xu G, Yang C, et al. Efficacy of the technique of piezoelectric corticotomy for orthodontic traction of impacted mandibular third molars. Br J Oral Maxillofac Surg. 2015;53(4):326-331.
59. Gantes B, Rathbun E, Anholm M. Effects on the periodontium following corticotomy-facilitated orthodontics. Case reports. J Periodontol. 1990;61(4):234-238.
60. Yaffe A, Fine N, Binderman I. Regional accelerated phenomenon in the mandible following mucoperiosteal flap surgery. J Periodontol. 1994;65(1):79-83.
61. Fischer TJ. Orthodontic treatment acceleration with corticotomy-assisted exposure of palatally impacted canines. Angle Orthod. 2007;77(3):417-420.
62. Twaddle BA, Ferguson DJ, Wilcko WM, et al. Dento-alveolar bone density changes following corticotomy-facilitated orthodontics [abstract]. J Dent Res. 2002;80:301.
63. Wilcko MT, Ferguson DJ, Makki L, Wilcko WM. Keratinized gingiva height increases after alveolar corticotomy and augmentation bone grafting. J Periodontol. 2015;86(10):1107-1115.
64. Owman-Moll P, Kurol J, Lundgren D. Continuous versus interrupted continuous orthodontic force related to early tooth movement and root resorption. Angle Orthod. 1995;65(6):395-401; discussion 401-402.
65. Harry MR, Sims MR. Root resorption in bicuspid intrusion. A scanning electron microscope study. Angle Orthod. 1982;52(3):235-258.
66. Ren A, Lv T, Kang N, et al. Rapid orthodontic tooth movement aided by alveolar surgery in beagles. Am J Orthod Dentofacial Orthop. 2007;131(2):160.e1-160.e10.
67. Cassetta M, Di Carlo S, Giansanti M, et al. The impact of osteotomy technique for corticotomy-assisted orthodontic treatment (CAOT) on oral health-related quality of life. Eur Rev Med Pharmacol Sci. 2012;16(12):1735-1740.
68. Chung KR, Oh MY, Ko SJ. Corticotomy-assisted orthodontics. J Clin Orthod. 2001;35(5):331-339.
69. Hajji S. The Influence of Accelerated Osteogenic Response on Mandibular De-Crowding [thesis]. St Louis, MO: St Louis University; 2000.
70. Makki L, Ferguson DJ, Wilcko MT, et al. Mandibular irregularity index stability following alveolar corticotomy and grafting: a 10-year preliminary study. Angle Orthod. 2015;85(5):743-749.
71. Wilcko MT, Wilcko WM, Murphy KG, et al. Full-thickness flap/subepithelial connective tissue grafting with intramarrow penetrations: three case reports of lingual root coverage. Int J Periodontics Restorative Dent. 2005;25(6):561-569.
72. Braut V, Bornstein MM, Belser U, Buser D. Thickness of the anterior maxillary facial bone wall—a retrospective radiographic study using cone beam computed tomography. Int J Periodontics Restorative Dent. 2011;31(2):125-131.
73. Wang HL, Boyapati L. “PASS” principles for predictable bone regeneration. Implant Dent. 2006;15(1):8-17.
74. Cassetta M, Pandolfi S, Giansanti M. Minimally invasive corticotomy in orthodontics: a new technique using a CAD/CAM surgical template. Int J Oral Maxillofac Surg. 2015;44(7):830-833.
75. Gkantidis N, Mistakidis I, Kouskoura T, Pandis N. Effectiveness of non-conventional methods for accelerated orthodontic tooth movement: a systematic review and meta-analysis. J Dent. 2014;42(10):1300-1319.
76. Han KH, Park JH, Bayome M, et al. Effect of frequent application of low-level laser therapy on corticotomized tooth movement in dogs: a pilot study. J Oral Maxillofac Surg. 2014;72(6):1182.e1-1182.e12.