You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
Head and neck cancer is the sixth most common cancer worldwide, resulting in ~ 640,000 cases and ~ 350,000 deaths per year. Most of these patients receive irradiation treatment.1 This treatment modality has a pronounced effect on the salivary glands, resulting in irreversible damage to the parenchymal tissue.2 Patients who have received irradiation treatment have rampant dental caries, frequent mucosal infections, and difficulties swallowing and chewing dry food. These patients are also sensitive to spicy food, have alterations in taste sensation and perception, and experience considerable pain originating in the impaired salivary glands, coughing episodes, voice disturbances, speech difficulties, and discomfort, all of which significantly decrease quality of life.3
Therapeutic Model
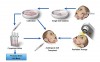
In recent years, the authors of this article and other researchers have concentrated on methods to restore normal salivary glands function in these patients.4,5 The notion of salivary gland regeneration is based on the assumption that autologous salivary gland graft cells can be isolated before initializing irradiation, cultivated, and preserved during the irradiation period. After treatment, cells are implanted into the irradiated gland and replace the functionally damaged cells (Figure 1).
Salivary Gland Stem Cells
The specific mechanisms involved in the physiological regeneration of salivary cells are still unknown, although regeneration from a putative noncommitted stem cell population is preferred.6 To date, the specific tissue stem cells remain unidentified and their exact location within the salivary glands remains obscure.7 Many nuclear, cytoplasmic, and cell surface markers were suggested to characterize salivary gland stem and progenitor cells (Table 1). Numerous studies hypothesize that salivary gland progenitor cells are located among the intercalated duct basal cells, as they have been shown to have a high proliferating capacity.6,8-11 These basal cells can migrate horizontally or luminally during differentiation (Figure 2). Other groups supporting this notion showed that tissue damage induced by ligation of the main salivary gland ducts leads to severe atrophy of the acini and granular ducts. The reopening of the ligated ducts results in marked proliferation of the ductal cells, which leads to repopulation of the acinar cell region.12,13 Integrin α6β1, which is part of the hemidesmosome complex and is responsible for the adhesion to extracellular laminin, has been suggested as a specific basal cell surface marker for isolation of salivary gland progenitor cells.13,14 The integrin α6β1–positive cell population was reported as possible progenitor cells mainly for liver and pancreatic lineages13 after isolating them 6 days for duct ligation, salivary gland removal, and cultivation. c-Kit (CD117) is a tyrosine kinase receptor, the expression of which is a common trait of stem and precursor cells in the organism, suggesting that it may also be a key in conferring and maintaining stemness and high self-renewal potency.15-17 The α6β1-positive cell population was shown to be positive for cell-surface markers such as Sca-1 and c-Kit as well.18
Several methods, including selection utilizing a growth matrix,13,19 specific growth conditions,20 and salispheres,1,5 have been described for isolation and culture of the salivary gland stem cells. As mentioned, salivary tissue consists of different cells, such as parenchymal cells, fibroblasts, and blood cells. Therefore, primary cultures of dispersed cells will always contain the entire repertoire of cell types, making it difficult to choose a specific salivary gland cell7 and will be restricted to a relatively limited culture lifespan during in vitro cultivation, resulting in a narrow time window for implantation. Previously, the authors of this article described a methodology for the isolation of salivary cells expressing integrin α6β1 from adult rodents using magnetic affinity cell sorting technology, which resulted in a very high-purity cell fraction of a putative progenitor cell population termed salivary gland integrin α6β1 positive cells (SGIE).21,22 Other research groups, such as Coppes et al, use different cell surface markers such as c-Kit, Sca-1, prominin-1, and integrin α6β1 to enrich the epithelial progenitor cells in the salivary glands.16
Irradiation Mechanism
Radiosensitivity of salivary gland acinar cells was considered for a long time an enigmatic behavior as these cells are nondividing and well differentiated with a structure and function similar to exocrine pancreas cells.2 Unlike other radiosensitive tissues, salivary gland cells proliferate slowly and are highly differentiated. Thus, the effect of irradiation cannot be attributed solely to rates of tissue proliferation. In 1984, Abok et al23 suggested that irradiation of salivary glands causes membrane damage to the secretion granules in the secreting cells. This results in proteolytic enzymes leakage, causing the cells to disappear. However, Konings et al2 reported in 2005 that after 15 Gy irradiation, no change was in the population of acinar cells both in parotid and submandibular glands a few days after irradiation, indicating that cell disappearance due to granular leakage is not substantial.2 One of the main theories suggests that the irradiation results in sublethal DNA damage, which manifests and becomes lethal at a delayed phase. Thus, when salivary progenitor cells are reproducing and parenchymal replenishment is needed, they die.24 DNA damage to progenitor cells and stem cells causes insufficient cell renewal.2,25
The authors examined the effects of irradiation on salivary glands26 in order to investigate and define the molecular mechanisms affecting salivary glands following irradiation, directing efforts on saliva proteome and global transcription profile of submandibular salivary gland (SSG) tissue. Saliva protein patterns were studied by proteomic analysis following mass spectrometry analysis that revealed proteins originating from SSG that were reduced in expression and proteins derived from the serum that exhibited increased expression, both indicating salivary tissue damage.26 The increase in serum proteins suggests irradiation damaged the extracellular environment, causing loss of barrier function between serum and salivary glands, whereas the decrease in salivary gland–derived proteins suggests damage to the protein synthesis, processing, or trafficking pathways. To examine whether irradiation causes alterations in mRNA expression, microarray analysis was performed, followed by validations. The authors found significant alterations in cell cycle–regulating genes in which 4 were found to be affected by irradiation: cyclin D1, p21, p57, and Ywhaq.26 Following irradiation, most of these genes showed a major up-regulation, which suggests that a cell cycle-arrest response occurs after irradiation.26 c-Kit–expressing cells have been suggested to belong to the salivary gland cell-dividing fraction.15 To further validate cell proliferation and cell arrest, the authors examined c-Kit transcription expression in vivo and the number of SGIE cell colonies21 obtained from the SSGs before and after irradiation. The authors found that they both were significantly decreased, indicating damage to the progenitor cells and stem cells, causing insufficient cell renewal and cells’ proliferation abilities.2,24,26
Salivary Gland Regeneration
At present, the major restriction in handling salivary gland stem cells, as also reported for c-Kit expressing salivary stem cells,15 is their relatively limited lifespan during in vitro cultivation, resulting in a narrow time window for implantation. To overcome this difficulty, the authors established a personal autologous salvary gland stem cell bank (Figure 1).27 Personal stem cell banking may be important for the repair process by autologous transplantation due to the long time frame (approximately 6 weeks or more) of the irradiation treatment that might result in reduced implantation efficiency. SGIE cells were cryopreserved for up to 3 years and evaluated for genetic and functional stability. No alterations were found, indicating no tumorigenic changes due to the cryopreservation–thawing–reculturing process.27
Another important issue is the safety of the implantation in regard to future clinical applications. Using cultures derived from a single cell rather than from cells originated from a mixture of cell clones enables better evaluation for whether a representative cell sample is expressing a tumor-related phenotype. For that reason, the authors created a novel methodology for the production of single cell–based salivary stem cell clones,27 which is essential for safety assurance of future clinical implantation.
Salivary gland stem cells were isolated from salisphere cultures and were transplanted into murine-irradiated SSGs.15,28 This transplantation resulted in 70% recovery of salivary flow rate, when implanted with 300 c-Kit donor cells.15 When non–c-Kit cells were implanted, only 33% recovery of salivary flow rate was reported. The regenerative capacity of other salivary gland stem cells-expressing markers such as α6β1, and CD133 was examined. The result was functional recovery of the saliva flow. Although these animal salivary models show promising results, confirmatory data in humans are required and further studies and technologic fine-tuning are needed before clinical implementation.
About the Authors
Raluca Stiubea-Cohen, MSc, PhD cand.
PhD student
Faculty of Dental Medicine
Institute of Dental Sciences
The Hebrew University of Jerusalem
Jerusalem, Israel
Ran David, MD, PhD
Faculty of Dental Medicine
Institute of Dental Sciences
The Hebrew University of Jerusalem
Jerusalem, Israel
Yoav Neumann, MSc, PhD cand.
PhD student
Faculty of Dental Medicine
Institute of Dental Sciences
The Hebrew University of Jerusalem
Jerusalem, Israel
Aaron Palmon, DMD, PhD
Professor
Faculty of Dental Medicine
Head, Institute of Dental Sciences
The Hebrew University of Jerusalem
Jerusalem, Israel
Doron J. Aframian, DMD, PhD
Associated Professor
Head, Salivary Gland Clinic and Saliva Diagnostic Laboratory
Department of Oral Medicine
The Hebrew University of Jerusalem
Jerusalem, Israel
Hadassah Medical Center
Jerusalem, Israel
References
1. Vokes EE, Weichselbaum RR, Lippman SM, Hong WK. Head and neck cancer. N Engl J Med. 1993;328(3):184-194.
2. Konings AW, Coppes RP, Vissink A. On the mechanism of salivary gland radiosensitivity. Int J Radiat Oncol Biol Phys. 2005;62(4):1187-1194.
3. Fox PC. Acquired salivary dysfunction. Drugs and radiation. Ann N Y Acad Sci. 1998;842:132-137.
4. Aframian DJ, Cukierman E, Nikolovski J, Mooney DJ, Yamada KM, Baum BJ. The growth and morphological behavior of salivary epithelial cells on matrix protein-coated biodegradable substrata. Tissue Eng. 2000;6(3):209-216.
5. Aframian DJ, David R, Ben-Bassat H, et al. Characterization of murine autologous salivary gland graft cells: a model for use with an artificial salivary gland. Tissue Eng. 2004;10(5-6):914-920.
6. Ihrler S, Zietz C, Sendelhofert A, Lang S, Blasenbreu-Vogt S, Löhrs U. A morphogenetic concept of salivary duct regeneration and metaplasia. Virchows Arch. 2002;440(5):519-526.
7. Coppes RP, Stokman MA. Stem cells and the repair of radiation-induced salivary gland damage. Oral Dis. 2011;17(2):143-153.
8. Man YG, Ball WD, Marchetti L, Hand AR. Contributions of intercalated duct cells to the normal parenchyma of submandibular glands of adult rats. Anat Rec. 2001;263(2):202-214.
9. Fonseca I, Moura Nunes JF, Soares J. Expression of CD44 isoforms in normal salivary gland tissue: an immunohistochemical and ultrastructural study. Histochem Cell Biol. 2000;114(6):483-488.
10. Schwartz-Arad D, Arber L, Arber N, Zajicek G, Michaeli Y. The rat parotid gland—a renewing cell population. J Anat. 1988;161:143-151.
11. Zajicek G, Schwartz-Arad D, Arber N, Michaeli Y. The streaming of the submandibular gland. II: Parenchyma and stroma advance at the same velocity. Cell Tissue Kinet. 1989;22(5):343-348.
12. Takahashi S, Schoch E, Walker NI. Origin of acinar cell regeneration after atrophy of the rat parotid induced by duct obstruction. Int J Exp Pathol. 1998;79(5):293-301.
13. Okumura K, Nakamura K, Hisatomi Y, et al. Salivary gland progenitor cells induced by duct ligation differentiate into hepatic and pancreatic lineages. Hepatology. 2003;38(1):104-113.
14. Franchi A, Santoro R, Paglierani M, Bondi R. Immunolocalization of alpha 2, alpha 5, and alpha 6 integrin subunits in salivary tissue and adenomas of the parotid gland. J Oral Path Med. 1994;23(10): 457-460.
15. Lombaert IM, Brunsting JF, Wierenga PK, et al. Rescue of salivary gland function after stem cell transplantation in irradiated glands. PLoS One. 2008;3(4):e2063.
16. Lombaert IM, Knox SM, Hoffman MP. Salivary gland progenitor cell biology provides a rationale for therapeutic salivary gland regeneration. Oral Dis. 2011;17(5):445-449.
17. Pittoni P, Piconese S, Tripodo C, Colombo MP. Tumor-intrinsic and extrinsic roles of c-Kit: mast cells as the primary off-target of tyrosine kinase inhibitors. Oncogene. 2011;30(7):757-769.
18. Hisatomi Y, Okumura K, Nakamura K, et al. Flow cytometric isolation of endodermal progenitors from mouse salivary gland differentiate into hepatic and pancreatic lineages. Hepatology. 2004;39(3):667-675.
19. Kishi T, Takao T, Fujita K, Taniguchi H. Clonal proliferation of multipotent stem/progenitor cells in the neonatal and adult salivary glands. Biochem Biophys Res Commun. 2006;340(2):544-552.
20. Kurth BE, Hazen-Martin DJ, Sens MA, Sens DA. Ultrastructural and immunohistochemical characterization of submandibular duct cells in culture and modification of outgrowth differentiation by manipulation of calcium-ion concentration. In Vitro Cell Dev Biol. 1988;24(6):593-600.
21. David R, Shai E, Aframian DJ, Palmon A. Isolation and cultivation of integrin alpha(6)beta(1)-expressing salivary gland graft cells: a model for use with an artificial salivary gland. Tissue Eng Part A. 2008;14(2):331-337.
22. Palmon A, David R, Neumann Y, Stiubea-Cohen R, Krief G, Aframian DJ. High-efficiency immunomagnetic isolation of solid tissue-originated integrin-expressing adult stem cells. Methods. 2012;56(2):305-309.
23. Abok K, Brunk U, Jung B, Ericsson J. Morphologic and histochemical studies on the differing radiosensitivity of ductular and acinar cells of the rat submandibular gland. Virchows Arch B Cell Pathol Incl Mol Pathol. 1984;45(4):443-460.
24. Nagler RM. The enigmatic mechanism of irradiation-induced damage to the major salivary glands. Oral Dis. 2002;8(3):141-146.
25. Coppes RP, Vissink A, Konings AW. Comparison of radiosensitivity of rat parotid and submandibular glands after different radiation schedules. Radiother Oncol. 2002;63(3):321-328.
26. Stiubea-Cohen R, David R, Neumann Y, et al. Effect of irradiation on cell transcriptome and proteome of rat submandibular salivary glands. PLoS One. 2012;7(7):e40636.
27. Neumann Y, David R, Stiubea-Cohen R, Orbach Y, Aframian DJ, Palmon A. Long-term cryopreservation model of rat salivary gland stem cells for future therapy in irradiated head and neck cancer patients. Tissue Eng Part C Methods. 2012;18(9):710-718.
28. Nanduri LS, Maimets M, Pringle SA, et al. Regeneration of irradiated salivary glands with stem cell marker expressing cells. Radiother Oncol. 2011;99(3):367-372.
*These two authors contributed equally to this work.