You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
Replacement of missing teeth with dental implants now ranks among the most successful procedures in dentistry,1 with millions of implants being placed annually. Achieving optimal esthetics, function, and phonetics with implant-supported restorations depends largely on the presence of adequate bone at the dental implant placement site.
It is well documented that following tooth extraction, reductions in both the alveolar ridge height and width typically occur. The processes of tissue modeling and remodeling have been investigated in both animals2 and humans,3,4 and the routine loss of ridge volume has been explained by the absence of daily stress/strain stimulus required for physiological maintenance of the bone anatomy.5 In the absence of site preservation following tooth extraction, significant tissue-contour loss occurs during the 3 months post-extraction, averaging 3 mm to 5 mm at 6 months.6,7 Horizontal bone resorption of up to 30% lingually and 56% buccally has been reported,8 with overall reduction in ridge width of up to 50% found 1 year after extraction.3
To reduce the dimensional ridge changes that occur during post-extraction healing, various site-preservation techniques have been proposed,9-13 and significant reductions of the dimensional changes have been reported.14,15 Detailed analysis of the 2003 Iasella study,14 for example, demonstrates that approximately 125% more loss of horizontal ridge width can be expected in the absence of site preservation (1.2-mm ridge loss with site preservation versus 2.6-mm ridge loss without site preservation), while up to 244% more loss of vertical ridge height can be expected with no site preservation (1.3-mm gain in ridge height with site preservation versus 0.9-mm loss of ridge height without site preservation). Previously published approaches for extraction site preservation include placement of allograft, xenograft, and alloplast graft materials, with or without the use of occlusive membranes. For allografts, both mineralized and demineralized bone have been utilized. However, no mixtures of mineralized and demineralized allograft have generally been commercially available until recently. One possible explanation for this is that US Food and Drug Administration (FDA) regulations require that all human bone allograft products be labeled with unique alphanumeric identification codes that allow each manufacturer to record and track the graft material to its recipient (and vice versa).16 Consequently, offering a product that includes bone from the same donor that has been processed in two different ways requires a somewhat more complicated manufacturing process.
The aim of this case series is to document the use of a relatively new bone allograft product comprised of blended mineralized and demineralized cortical bone for extraction site preservation procedures.
Case Series Treatment
Ten consecutive patients who had sought treatment at a private practice limited to periodontics and dental implants in Austin, Texas, were identified. All patients had required extraction of a tooth with site preservation to facilitate future placement of dental implants. Written and signed informed consent was obtained prior to treatment for all patients. Local anesthesia was obtained using 4% articaine hydrochloride with 1:100,000 epinephrine (Septodont, www.septodontusa.com). In all cases, a full-thickness mucoperiosteal flap was reflected, and tooth removal was accomplished atraumatically. Multi-rooted teeth were sectioned with a high-speed handpiece, while single-rooted teeth were removed with periotome assistance.
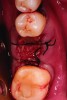
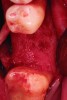
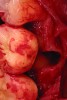
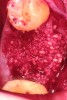
Upon removal of the tooth, sockets were thoroughly degranulated with hand instruments and irrigated with sterile saline. Each socket was then filled with CREOS 70/30 bone allograft (Nobel Biocare, www.nobelbiocare.com) and covered with a single layer of amnion-chorion (Snoasis Medical Products, www.snoasismedical.com). No primary closure was attempted in any cases of this report. In cases with significant osseous defects such as a missing buccal plate, a secondary collagen membrane (Community Tissue Services™, www.communitytissue.org) was utilized for graft containment at the buccal wall, and an amnion-chorion barrier was perpendicularly layered over the collagen membrane. The purpose of the amnion-chorion membrane in all cases, whether used alone or in conjunction with a collagen barrier, was to act as a matrix for rapid epithelial cell migration over the non-primary closure of the socket. Prior to placement, the bone allograft was only hydrated with sterile saline, and no growth factors were used or mixed with the bone. To reiterate, in all cases, primary closure was intentionally not achieved.
Post-surgically, all patients were prescribed antibiotics depending on their various individual drug allergies and a combination of analgesic and narcotic medications for pain. Oral rinses such as chlorhexidine were not prescribed following surgery, and patients were instructed to brush and floss normally. Patients returned for an initial follow-up visit 10 days post surgery, at which time sutures were removed and teeth were cleaned with hand instruments. Six weeks following surgery, patients were seen for an interim visit that included radiographic and clinical examination of the surgical site.
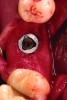
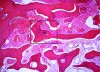
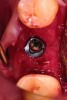
Twelve to 16 weeks after the initial surgery (average 14.2 weeks), each patient presented for dental implant placement. While preparing the previously grafted sites for implant insertion, a 2-mm trephine core sample was taken from each surgical site prior to completion of the final drilling sequence. The trephined bone cores were submitted to the University of Missouri–Kansas City School of Dentistry for histologic examination.
Results
This article reports on treatment of 10 patients with a new blended bone allograft material that was placed in fresh extraction sockets to preserve the alveolar ridge in anticipation of subsequent implant placement. A total of six females and four males with an average age of 54.6 years (± 6.2 years) were included in this case series. Two of the patients were smokers, and one patient was a well-controlled diabetic. All other patients had relatively unremarkable medical and social histories. The 10 surgical sites consisted of three maxillary molars, three mandibular molars, two maxillary premolars, one mandibular premolar, and one maxillary central incisor. All 10 surgical sites healed uneventfully with no incidences of infection, graft loss, or other complications.
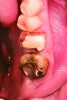
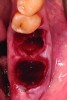
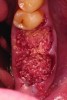
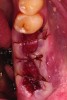
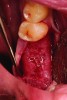
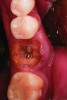
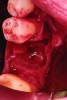
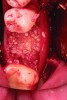
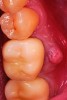
After roughly 3 months (14.2 weeks), bone formation in all cases was adequate in both buccolingual and apicocoronal dimensions to enable proper locational placement of the planned dental implants without the need for any additional bone-graft material. Figure 1 through Figure 19 depict three of the cases. Analysis of the trephine core samples (Table 1) revealed results consistent with findings of previously published site-preservation studies.
Discussion
Bone allografts have been used in dentistry for over 40 years, with more than 800,000 transplantations performed annually in the United States, a more than 400% increase since 1972.17 Rigorous donor screening and aseptic proprietary processing programs have rendered their use safe and effective.16 All harvested tissue is routinely tested for human immunodeficiency virus (HIV), hepatitis B and C, human T-lymphotropic virus, as well as bacterial and fungal contaminants. Once harvested, the bone is processed in hyper-clean facilities that strictly control temperature, humidity, ionization, electrostatic discharge, air pressure, air ventilation, and air filtration.16 Soft tissue is stripped away, and the hard tissue is then sectioned to manageable sizes, rigorously cleansed, and decontaminated. Nearly all of the moisture content is eliminated via lyophilization or repetitious solvent baths that reduce antigenicity and enable long shelf storage of up to 3 years at room temperature.
The particle size of mineralized bone allograft typically ranges between 250 to 1,000 micrometers. Once reduced and packaged, additional low-dose irradiation may also be administered for terminal sterilization of the graft. In the case of demineralized bone allograft, the bone is typically immersed in a hydrochloric acid bath for various lengths of time in an effort to demineralize the bone by reducing calcium content. The bone is then washed in various proprietary buffer solutions to remove residual acid prior to terminal processing with low-dose gamma irradiation. These tissue processes vary depending on the proprietary formulation of individual bone banks. The bone allograft used in this particular case series was processed via the patented Allowash® (LifeNetHealth, www.accesslifenethealth.org) process and had a particle size of 250 to 1,000 micrometers. Additionally, it was a blended ratio of 70:30 mineralized to demineralized cortical bone from the same individual donors.
Multiple studies have evaluated the bone-forming effectiveness of both mineralized and demineralized bone allografts when used for dental surgery.18-24 Different mechanisms are at work in the two types of graft materials.25 Demineralized bone allograft, for example, provides a source of osteoinductive factors.26 It contains type I collagen and various proteins such as bone morphogenetic protein (BMP) with the capacity to influence cell behavior, including proliferation and attachment.27 Shigeyama and colleagues tested commercially prepared demineralized bone allograft via multiple assays and Western Blot analysis, with results showing the allograft retained proteins such as BMPs 2, 4, and 7.27 By reducing the calcium content of allograft particles via demineralization, it is thought that faster release of residual proteins such as BMPs may be attained for improved bone healing. Multiple studies have documented higher osteogenic capacity in vitro for demineralized bone allograft compared to mineralized bone allograft.18,19 Accordingly, one recent study that compared demineralized to mineralized bone allograft used in extraction socket site preservation found significantly greater new bone formation from the use of the demineralized material.28 In this study, however, it is interesting to note that no significant differences in alveolar ridge dimensional changes were noted between the two allograft groups and that only sockets with minimal dehiscence defects were included in the study. The latter statement highlights one of the problems that many clinicians find with demineralized bone allograft. Because the product is demineralized, its structural integrity is minimized. While this is beneficial for the rapid release of BMPs, it may pose a problem in osseous defects that are not well contained, such as extraction sockets with significant dehiscences or absent bony walls. The issues associated with allograft structural integrity are minimized with mineralized products. Because they retain their calcium content, mineralized cortical allografts are considered osteoconductive materials29 and are better suited for defects with less than optimal containment, especially when compared with demineralized allografts.30
The availability of a product that combines both demineralized and mineralized bone allograft allows the benefits of both to be obtained. Because the cases in this series were consecutive, there were no exclusionary criteria, and some of the cases treated had significant osseous defects that compromised graft containment. The use of a blended bone allograft to treat these cases provided stability from the osteoconductive capacity of the mineralized cortical component with the additional benefit of improved osteoinductive capacity from the demineralized component.
Notably, while the findings from this case series compare favorably to previously published extraction site preservation studies, some important and significant differences exist. First, the surgical sites in this case series were intentionally left exposed without primary closure. Second, the time allowed for osseous healing from the time of extraction site preservation to the placement of dental implants was shorter than most other studies. These findings suggest that the use of a blended bone allograft product for extraction site preservation procedures has the potential to produce favorable results for the facilitation of future dental implant placement, even in compromised situations.
Conclusion
This case series suggests that excellent results can be obtained when using a blended mineralized-demineralized cortical bone allograft product to preserve alveolar ridge dimensions after tooth extraction. The findings of this case series warrant additional controlled studies with expanded patient populations to confirm these findings.
DISCLOSURE
The author has no affiliation with any of the products mentioned in this article.
ABOUT THE AUTHOR
Dan Holtzclaw, DDS, MS
Private Practice; Austin, Texas
Queries to the author regarding this course may be submitted to authorqueries@aegiscomm.com.
REFERENCES
1. Brown LJ, Babbush CA. The future need and demand for dental implants. In: Babbush CA, Hahn JA, Krauser JT, Rosenlicht JL, eds. Dental Implants: The Art and Science. 2nd ed. Maryland Heights, MO: Saunders Elsevier; 2011:1-16.
2. Araújo MG, Lindhe J. Dimensional ridge alterations following tooth extraction. An experimental study in the dog. J Clin Periodontol. 2005;32(2):212-218.
3. Amler MH, Johnson PL, Salman I. Histological and histochemical investigation of human alveolar socket healing in undisturbed extraction wounds. J Am Dent Assoc. 1960;61:32-44.
4. Pietrokovski J, Massler M. Alveolar ridge resorption following tooth extraction. J Prosthet Dent. 1967;17(1):21-27.
5. Hansson S, Halldin A. Alveolar ridge resorption after tooth extraction: A consequence of a fundamental principle of bone physiology. J Dent Biomech. 2012;3. doi:10.1177/1758736012456543.
6. Lam RV. Contour changes of the alveolar process following extractions. J Prosthet Dent. 1960;10(1):25-32.
7. Schropp L, Wenzel A, Kostopoulos L, Karning T. Bone healing and soft tissue contour changes following single-tooth extraction: a clinical and radiographic 12-month prospective study. Int J Periodontics Restorative Dent. 2003;23(4):313-323.
8. Botticelli D, Berglundh T, Lindhe J. Hard-tissue alterations following immediate implant placement in extraction sites. J Clin Periodontol. 2004;31(10):820-828.
9. Tarnow DP, Eskow RN, Zamzok J. Aesthetics and implant dentistry. Periodontol 2000. 1996;11:85-94.
10. Nevins M, Mellonig JT. Enhancement of the damaged edentulous ridge to receive dental implants: a combination of allograft and the GORE-TEX membrane. Int J Periodontics Restorative Dent. 1992;12(2):96-111.
11. Mellonig JR, Triplett RG. Guided tissue regeneration and endosseous dental implants. Int J Periodontics Restorative Dent. 1993;13(2):108-119.
12. Simion M, Dahlin C, Trisi P, Piatelli A. Qualitative and quantitative comparative study on different filling materials used in bone tissue regeneration: a controlled clinical study. Int J Periodontics Restorative Dent. 1994;14(3):198-215.
13. Lekovic V, Kenney EB, Weinlaender M, et al. A bone regenerative approach to alveolar ridge maintenance following tooth extraction. Report of 10 cases. J Periodontol. 1997;68(8):563-570.
14. Iasella JM, Greenwell H, Miller RL, et al. Ridge preservation with freeze-dried bone allograft and a collagen membrane compared to extraction alone for implant site development: a clinical and histologic study in humans. J Periodontol. 2003;74(7):990-999.
15. Nevins M, Camelo M, De Paoli S, et al. A study of the fate of the buccal wall of extraction sockets of teeth with prominent roots. Int J Periodontics Restorative Dent. 2006;26(1):19-29.
16. Holtzclaw D, Toscano N, Eisenlohr L, Callan D. The safety of bone allografts used in dentistry: a review. J Am Dent Assoc. 2008;139(9):1192-1199.
17. Allograft safety and ethical considerations. Proceedings of the fourth symposium sponsored by the Musculoskeletal Transplant Foundation. September 2003. Edinburgh, Scotland, United Kingdom. Clin Ortho Relat Res. 2005;435:2-117.
18. Mellonig JT, Bowers GM, Bailey RC. Comparison of bone graft materials. Part I. New Bone formation with autografts and allografts determined by Strontium-85. J Periodontol. 1981;52(6):291-296.
19. Mellonig JT, Bowers GM, Cotton WR. Comparison of bone graft materials. Part II. New bone formation with autografts and allografts: a histological evaluation. J Periodontol. 1981;52(6):297-302.
20. Toloue SM, Chesnoiu-Matei I, Blanchard SB. A clinical and histomorphometric study of calcium sulfate compared with freeze-dried bone allograft for alveolar ridge preservation. J Periodontol. 2012;83(7):847-855.
21. Froum S, Cho SC, Rosenberg E, et al. Histological comparison of healing extraction sockets implanted with bioactive glass or demineralized free-dried bone allograft: a pilot study. J Periodontol. 2002;73(1):94-102.
22. Block MS, Finger I, Lytle R. Human mineralized bone in extraction sites before implant placement: preliminary results. J Am Dent Assoc. 2002;133(12):1631-1638.
23. Wang HL, Tsao YP. Histologic evaluation of socket augmentation with mineralized human allograft. Int J Periodontics Restorative Dent. 2008;28(3):231-237.
24. Fugazzotto PA, Shanaman R, Manos T, Shectman R. Guided bone regeneration around titanium implants: report of the treatment of 1,503 sites with clinical reentries. Int J Periodontics Restorative Dent. 1997;17(3):292-299.
25. Committee on Research, Science and Therapy of the American Academy of Periodontology. Tissue Banking of bone allografts used in periodontal regeneration. J Periodontol. 2001;72(6):834-838.
26. Urist MR. Bone: formation by autoinduction. Science. 1965;150(3698):893-899.
27. Shigeyama Y, D’Errico JA, Stone R, Somerman MJ. Commercially-prepared allograft material has biological activity in vitro. J Periodontol. 1995;66(6):478-487.
28. Wood RA, Mealey BL. Histologic comparison of healing after tooth extraction with ridge preservation using mineralized versus demineralized free-dried bone allograft. J Periodontol. 2012;83(3):329-336.
29. Rosen PS, Reynolds MA. A retrospective case series comparing the use of demineralized freeze-dried bone allograft and freeze-dried bone allograft combined with enamel matrix derivative for the treatment of advanced osseous lesions. J Periodontol. 2002;73(8):942-949.
30. Cammack GV II, Nevins M, Clem DS III. Histologic evaluation of mineralized and demineralized freeze-dried bone allograft for ridge and sinus augmentations. Int J Periodontics Restorative Dent. 2005;25(3):231-237.