You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
Successful, osseointegrated dental implants are surrounded by three different tissues: epithelium, connective tissue, and bone. Long-term stability of dental implants depends on the integration of the implants with these tissues. Maintaining healthy soft- and hard-tissue–implant interfaces is believed to be critical to long-term implant survival.1,2 It has been suggested that a certain width of peri-implant tissues (ie, the biologic width) is required to enable an optimal epithelial/connective-tissue attachment to the implant. If soft-tissue attachment levels are not satisfactory, bone resorption will occur until the optimal biologic width is established.3-8
The use of restorative components one size smaller than implant-restorative platforms (so-called platform-switching) has resulted in a paradigm shift relative to establishment and creation of the biologic width.9 Cochran et al reported on the results of an in-vivo study in dogs that used matching- and smaller-diameter restorative components for both submerged and nonsubmerged implants.7 The biologic response to the platform-switched implants appeared to differ fundamentally from that found around the platform-matched ones, with the connective tissue around the former extending coronally past the implant–abutment junction (the microgap). The authors suggested that marginal inflammation had been eliminated or greatly reduced for the platform-switched connections.
Although the success rate of dental implants is high, some implants fail to osseointegrate. Functional failure may have multiple causes, including less-than-optimal surgical technique, inadequate treatment planning, pre-existing infection, patient inability to heal from osseous surgery, and lack of bone–implant contact prior to loading. After loading, factors associated with non-integrated implants may include occlusal overload, prosthetic design, and prosthetic misfit. Soft-tissue fibers attached to implants serve as barriers to epithelial migration; they minimize bacterial invasion in and around implant/bone/soft-tissue interfaces.10 Oral microflora around implants has been reported to be similar to oral flora around natural teeth.11 Pathogenic bacteria typically seen in patients with periodontitis may also contribute to implant failures.12 Cortelli et al reported that bacterial frequency increased from peri-implant/periodontal health to peri-implantitis/periodontitis, but not from mucositis/gingivitis to peri-implantitis/periodontitis.13 They found a trend towards greater bacterial frequency around teeth than around implants.
Biologic Adhesion
It has been well established that the initial event in the development of most bacterial diseases is adhesion of bacteria to biologic or artificial surfaces. Biofilms that form on teeth have been described as dental plaque. Biofilms in the oral cavity consist of complex microbial colonies embedded in a matrix of polymers primarily derived from bacteria and saliva. Bacteria in dental plaque are the major etiologic factors in the development of dental caries, gingivitis, periodontitis, and peri-implantitis. Biofilms on the surfaces of dental implants have been described as the main source of pathogens causing peri-implantitis, a disease described as one of the main causes of dental implant loss.14 Therefore, preventing bacterial adhesion to either intraoral hard or soft tissues has been considered to be an important parameter of clinical success/survival.
A strong in-vivo correlation has been reported between the number of bacteria adhering to periodontal epithelium and the degree of inflammation.15 Bacterial adherence to implants has also been considered to be an important part of the development of peri-implant disease. Implant surfaces generally come into contact with oral fluids, including saliva and fluids in the gingival and peri-implant sulci. The mechanisms by which oral bacteria adhere to solid surfaces are not fully understood. In the oral cavity, an acquired pellicle attaches to dental implant surfaces. The pellicle is formed by adsorption of salivary components to the surface of transgingival abutments. Oral bacteria must then interact with these biologic adhesions in order to adhere to the surface. Wu-Yuan et al reported that bacterial attachments to implant surfaces were directly related to the surface roughness of the implants. More bacteria attached to rough implant surfaces, while smooth-surfaced implants demonstrated poor attachment of bacteria.16 Both surface characteristics and chemical composition of implant surfaces affect the accumulation of dental plaque.
Titanium-Nitride (TiN) Coating of Dental Implants and Abutments
Titanium nitride (TiN) has been used as a coating to enable surgical materials to better resist wear and corrosion. Physical vapor deposition (PVD) is the most common method of depositing TiN on orthopedic implants. This process produces a stable layer on implant surfaces that changes the chemical composition of the surface. The TiN is formed by the reaction of pure titanium and nitrogen gas in a vapor phase before deposition.17,18 The thickness of nitride coatings has been reported to be 1 to 5 microns. Properties of TiN coatings interfere with and absorb white light to give the appearance of a gold color.
Various reports have characterized TiN as having a high degree of chemical inertness, a low friction coefficient, and optimal biocompatibility.19-21 In the medical field, TiN has been used to reduce attrition coefficients and increase the hardness and corrosion resistance of surfaces, thus reducing interaction of biological liquids with metal bases.22 Scarano et al reported that TiN surfaces demonstrated a significant reduction in the number of bacteria.23 The authors thought this finding could be important for decreasing inflammation of peri-implant soft tissues.
Quirynen et al studied the effect of abutment surface roughness on bacterial adhesion and biofilm formation; they found that reductions in surface smoothness (less than 0.2 micron roughness) had no major effect on quantitative or qualitative microbiologic adhesions, colonization, or microbiologic composition—supra- or subgingivally.24 This finding was replicated in a longer-term study.25 The researchers concluded that below a threshold roughness, no further impact on bacterial adhesion and/or colonization should be expected. Grossner-Schreiber et al compared bacterial colony formation on titanium implant surfaces modified with TiN and zirconium nitride (ZrN) with that on polished titanium surfaces.26 They found significantly less bacterial adhesion and plaque formation on the modified surfaces, a finding that was later confirmed.23
Zirconia Versus Titanium Abutment Fracture Resistance
One of the major disadvantages of tooth-colored ceramic abutments and crowns is the potential for abutment fracture during insertion and/or occlusal loading. In a retrospective study, Koenig et al27 studied the performance of 147 zirconia tooth- and implant-supported crowns and fixed partial dentures (FPDs) in function for a mean period of 41.5 months. They reported that the survival rate of crowns and FPDs was 93.2%, the success rate was 81.63%, and the 9-year Kaplan-Meier estimated success rate was 52.66%. The chipping rate was 15%, and the framework fracture rate was 2.7%. Most fractographic analyses revealed that veneer fractures originated from occlusal surface roughness. Several parameters were shown to significantly influence veneer fracture, including: the absence of occlusal nightguards (P = 0.0048); the presence of ceramic restorations as antagonists (P = 0.013); parafunctional activity (P = 0.018); and implants as support for the restorations (P = 0.026). The implant abutment success rate was 100%. In a laboratory study specific to implant abutments, Sqhaireen reported that the highest fracture loads were associated with metal-ceramic crowns supported by titanium abutments (P = 0.000).28 IPS Empress® (Ivoclar Vivadent, www.ivoclarvivadent.com) crowns supported by zirconia abutments had the lowest fracture loads (P = 0.000). Fracture modes of metal-ceramic crowns supported by titanium abutments included screw fracture and screw bending. Fracture of both the crown and abutment was the dominant mode of failure of In-Ceram® (VITA, www.vita-zahnfabrik.com)/IPS Empress crowns supported by zirconia abutments. Sqhaireen also noted that failure modes of restorations supported by zirconia abutments were more catastrophic than those for restorations supported by titanium abutments.
Intraoral Shade and Color Matching Related to Abutment Materials
Replacement of missing teeth, soft tissues, and alveolar bone in the esthetic zone is one of the most challenging situations facing clinicians, laboratory technicians, and patients. Achieving optimal esthetic and functional results requires thorough and accurate diagnostic procedures and evaluations; this process includes the selection of appropriate grafting materials, if needed, and a definitive implant system. The surgical and prosthetic components of a given implant system may have important influences on the esthetics of the restorations.29
While all-ceramic tooth- and implant-supported restorations have become increasingly prevalent, the esthetic results of all-ceramic restorations cemented over metallic implant abutments may be questionable. Sailer et al recommended that when choosing abutments for anterior single-unit cases, several factors should be considered, including smile-line height, soft-tissue thickness, the color of the neighboring teeth, and the patient’s esthetic expectations.30 For esthetically demanding situations, customized ceramic abutments were recommended. For patients with thin peri-implant soft tissues, a combination of zirconia abutments and all-ceramic crowns were recommended. In cases with thick mucosa, titanium abutments could be used, when combined with metal-ceramic crowns. To avoid difficulties with retained cement, Sailer et al also recommended the use of screw-retained restorations whenever screw-access openings could be positioned palatal to the incisal edges of the implant restorations.
The abutment color may show through peri-implant soft tissues and affect the overall appearance and esthetics of restorations. In a recent study, Jung et al found that all-ceramic restorations matched the color of the unrestored neighboring teeth significantly better than porcelain-fused-to-metal restorations on titanium or gold abutments.31
Bressan et al studied the influence of abutment materials on peri-implant soft-tissue color and concluded that no matter which type of restorative material was selected (gold, titanium, or zirconia), some differences between the color of the peri-implant soft tissue and the soft-tissue colors around natural teeth were discernible via spectrophotometric measurement.32 However, significantly higher differences were noted for titanium (silver-metallic) abutments than gold or zirconia ones. The thickness of peri-implant soft tissues did not appear to be a significant factor in any of the color differences.
The following case report illustrates the clinical results obtained with a gold-colored, titanium-nitride–coated abutment and platform-switched implant placed in the anterior maxilla.
Case Report
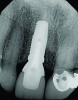
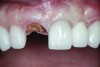
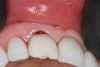
A 63-year-old female patient presented with mobility of her right maxillary incisor, tooth No. 8, secondary to endodontic therapy (Figure 1). Her medical and dental histories were non-contributory. Clinical and radiographic evaluations revealed an 8-mm probing depth on the palatal aspect. The tooth was deemed to be fractured (Figure 2). The treatment plan accepted by the patient was for extraction, immediate implant placement, and immediate provisional restoration, if possible.
Without reflecting a full-thickness mucoperiosteal flap, the root tip was carefully extracted using periotomes to preserve the buccal plate and interproximal bone (Figure 3 and Figure 4). The extraction site was carefully debrided, and an osteotomy was prepared following the manufacturer’s instructions for placement of a 5/4mm D x 13mm L OSSEOTITE® Tapered Certain® PREVAIL® Implant (BIOMET 3i, www.biomet3i.com).
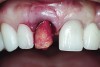
An osteotomy that used the dense cortical bone on the palatal aspect of the extraction socket was prepared, and a guide pin was inserted (Figure 5) to verify that the angulation was ideal. The implant was then inserted (Figure 6). Figure 7 shows the occlusal view of the implant in position. Note the gap between the palatally oriented implant and the buccal plate of the extraction socket.
A cover screw was placed on the implant, and a combination of allograft/xenograft was inserted into the gap between the implant and the osteotomy (Figure 8). A temporary cylinder (PreFormance® Temporary Cylinder, BIOMET 3i) was then placed into the implant and modified (Figure 9). The screw-access opening was blocked out. A preoperative polyvinylsiloxane (PVS) impression was filled with bis-GMA resin (Luxatemp®, DMG America, www.dmg-america.com) and inserted over the modified temporary cylinder (Figure 10). This was allowed to set for 2 minutes. The screw was removed, and the temporary cylinder and provisional crown were removed from the mouth. A healing abutment (EP® Healing Abutment, BIOMET 3i) was placed into the implant and hand-tightened to support the peri-implant soft tissue while the provisional restoration was being modified extraorally.
The temporary cylinder/provisional restoration complex was placed on a laboratory holder (Figure 11). The margins of the provisional restoration were modified by adding additional resin to the subgingival area between the gingival margin of the restoration and the implant restorative platform. Using flowable composite resin, all voids were filled, and the contours were otherwise refined to achieve an ideal emergence profile (consistent with the cross-sectional anatomy of a maxillary central incisor) during the healing phase. The gingival profile was intentionally left flat to prevent excessive pressure from being imposed on the facial gingival tissue. The provisional crown was then polished.
The crown was inserted intraorally and secured with an abutment screw that was torqued to 20 Ncm (Figure 12). The occlusion was adjusted so that the restoration had no contacts in excursive and functional movements (Figure 13). A cotton pellet was placed, and the access opening was restored with flowable composite resin. The occlusion was evaluated again to ensure that the patient could not load the implant restoration with any mandibular movements.
Six months later, the patient returned for fabrication of the definitive restoration. The provisional restoration was removed, and the implant was evaluated. It exhibited no mobility, and the peri-implant soft tissues had healed consistent with the shape of the provisional crown’s emergence profile. A pick-up implant impression coping was seated into the implant hex for an open-tray impression. The impression was made with heavy- and light-body PVS impression materials (Imprint™ 3, 3M ESPE, www.3MESPE.com).
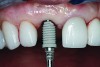
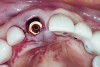
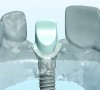
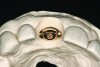
The implant impression, along with a cast of the opposing arch and shade, were sent to a commercial dental laboratory for fabrication of a master cast. A signed work order and the master cast were then sent to the BellaTek® Production Center (BIOMET 3i) for a lab-designed abutment (LDA) (Figure 14). A BellaTek® abutment was milled from a solid blank of titanium, and a gold-colored titanium-nitride coating was applied to impart a warm color through the thin marginal peri-implant tissues.
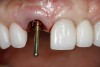
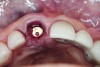
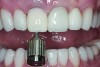
The master cast and abutment were returned to the laboratory for fabrication of the restoration (Figure 15). An all-ceramic crown (e.max®, Ivoclar Vivadent) was fabricated on the custom abutment; it matched the contours of the adjacent central incisor. After completion of custom shading, the definitive abutment and crown were returned to the restorative dentist. The abutment was tried in (Figure 16), and a radiograph was taken to confirm complete seating between the abutment/implant and the crown/abutment. The definitive abutment screw was torqued to 20 Ncm, and the screw-access opening was blocked out with Teflon tape.
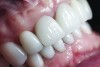
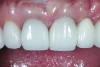
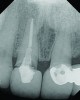
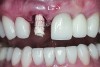
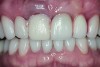
The crown was inserted and secured with a luting composite (Multilink® Automix, Ivoclar Vivadent) (Figure 17); a radiograph confirmed complete removal of excess cement (Figure 18). At the 4-month follow-up appointment, the soft tissues were healed within normal limits, and the patient was pleased with the esthetic outcome of the definitive restoration (Figure 19 and Figure 20).
Conclusion
Preventing bacterial adhesion to intraoral hard and/or soft tissues may help prevent the development of peri-implant disease, enhancing the likelihood of implant survival and long-term esthetic success. Surface characteristics and chemical composition of implant component surfaces both may have effects on the accumulation of dental plaque. Titanium nitride-coated surfaces have been associated with reduced bacterial loads. Furthermore, when TiN-coated abutments are used to support all-ceramic restorations, the gold color offers esthetic advantages. The case report presented in this article comprehensively illustrated use of such an abutment supported by a platform-switched implant placed in the anterior maxilla to achieve an excellent functional and esthetic result.
DISCLOSURE
Dr. Ramsey is a consultant and speaker for BIOMET 3i but did not receive compensation for this article. The authors have no other affiliation with any other manufacturers mentioned in this article.
ABOUT THE AUTHORS
Christopher D. Ramsey, DMD
Private Practice, Jupiter, Florida
Karina F. Leal, DMD
Private Practice, West Palm Beach, Florida
Queries to the author regarding this course may be submitted to authorqueries@aegiscomm.com.
REFERENCES
1. Hoshaw SJ, Brunski JB, Cochran GVB. Mechanical loading of Brånemark implants affects interfacial bone modeling and remodeling. Int J Oral Maxillofac Implants. 1994;9(3):345-360.
2. Adell R, Lekholm U, Rockler B, Brånemark PI. A 15-year study of osseointegrated implants in the treatment of the edentulous jaw. Int J Oral Surg. 1981;10(6):387-416.
3. Abrahamsson I, Berglundh T, Wennström J, Lindhe J. The peri-implant hard and soft tissues at different implant systems. A comparative study in the dog. Clin Oral Implant Res. 1996;7(3):212-219.
4. Abrahamsson I, Berglundh T, Glantz PO, Lindhe J. The mucosal attachment at different abutments. An experimental study in dogs. J Clin Periodontol. 1998;25(9):721-727.
5. Abrahamsson I, Berglundh T, Lindhe J. Soft tissue response to plaque formation at different implant systems. A comparative study in the dog. Clin Oral Implant Res. 1998;9(2):73-79.
6. Berglundh T, Lindhe J. Dimension of the periimplant mucosa. Biological width revisited. J Clin Periodontol. 1996;23(10):971-973.
7. Cochran DL, Mau LP, Higginbottom FL, et al. Soft and hard tissue histologic dimensions around dental implants in the canine restored with smaller-diameter abutments: a paradigm shift in peri-implant biology. Int J Oral Maxillofac Implants. 2013;28(2):494-502.
8. Cochran DL, Nevins M. Biologic width: a physiologically and politically resilient structure. Int J Periodontics Restorative Dent. 2012;32(4):371-373.
9. Lazzara RJ, Porter SS. Platform switching: a new concept in implant dentistry for controlling postrestorative crestal bone levels. Int J Periodontics Restorative Dent. 2006;26(1):9-17.
10. Ruggeri A, Franchi M, Trisi P, Piattelli A. Histologic and ultrastructural findings of gingival circular ligament surrounding osseointegrated nonsubmerged loaded titanium implants. Int J Oral Maxillofac Implants. 1994;9(6):636-643.
11. Apse P, Ellen RP, Overall CM, Zarb GA. Microbiota and crevicular fluid collagenase activity in the osseointegrated dental implant sulcus: a comparison of sites in edentulous and partially edentulous patients. J Periodontal Res. 1989;24(2):96-105.
12. Ong ES, Newman HN, Wilson M, Bulman JS. The occurrence of periodontitis-related microorganisms in relation to titanium implants. J Periodontol. 1992;63(3):200-205.
13. Cortelli SC, Cortelli JR, Romeiro RL, et al. Frequency of periodontal pathogens in equivalent peri-implant and periodontal clinical statuses. Arch Oral Biol. 2013;58(1):67-74.
14. Paquette DW, Brodala N, Williams RC. Risk factors for endosseous dental implant failure. Dent Clin North Am. 2006;50(3):361-374.
15. Vaahtoniemi LH, Räisänen S, Stenfors LE. Attachment of bacteria to oral epithelial cells in vivo: a possible correlation to gingival health status. J Periodontal Res. 1993;28(4):308-311.
16. Wu-Yuan CD, Eganhouse KJ, Keller JC, Walters KS. Oral bacterial attachment to titanium surfaces: a scanning electron microscopy study. J Oral Implantol. 1995;21(3):207-213.
17. Pappas MJ, Makris G, Buechel FF. Titanium nitride ceramic film against polyethylene. A 48 million cycle wear test. Clin Orthop Relat Res. 1995;(317):64-70.
18. Peterson CD, Hillberry BM, Heck DA. Component wear of total knee prostheses using Ti-6Al-4V, titanium nitride coated Ti-6Al-4V, and cobalt-chromium-molybdenum femoral components. J Biomed Mater Res. 1988;22(10):887-903.
19. Scarano A, Piattelli M, Vrespa G, et al. Bone healing around titanium and titanium nitride-coated dental implants with three different surfaces: an experimental study in rats. Clin Implant Dent Relat Res. 2003;5(2):103-111.
20. Dion I, Baquey C, Candelon B, Monties JR. Hemocompatibility of titanium nitride. Int J Artif Organs. 1992;15(10):617-621.
21. Galante JO, Lemons J, Spector M, et al. The biologic effects of implant materials. J Orthop Res. 1991;9(5):760-775.
22. Harman MK, Banks SA, Hodge WA. Wear analysis of a retrieved hip implant with titanium nitride coating. J Arthroplasty. 1997;12(8):938-945.
23. Scarano A, Piattelli M, Vrespa G, et al. Bacterial adhesion on titanium nitride-coated and uncoated implants: an in vivo human study. J Oral Implantol. 2003;29(2):80-85.
24. Quirynen M, Bollen CM, Papaioannou W, et al. The influence of titanium abutment surface roughness on plaque accumulation and gingivitis: short-term observations. Int J Oral Maxillofac Implants. 1996;11(2):169-178.
25. Bollen CM, Papaioanno W, Van Eldere J, et al. The influence of abutment surface roughness on plaque accumulation and peri-implant mucositis. Clin Oral Implants Res. 1996;7(3):201-211.
26. Grössner-Schreiber B, Griepentrog M, Haustein I, et al. Plaque formation on surface modified dental implants. An in vitro study. Clin Oral Implants Res. 2001;12(6):543-551.
27. Koenig V, Vanheusden AJ, Le Goff S, Mainjot AK. Clinical risk factors related to failures with zirconia-based restorations: an up to 9-year retrospective study. J Dent. 2013;41(12):1164-1174.
28. Sqhaireen MG. Fracture resistance and mode of failure of ceramic versus titanium abutments and single implant-supported restorations. Clin Implant Dent Relat Res. 2013 Oct 9. doi:10.1111/cid.12160. [Epub ahead of print]
29. Tarnow DP, Eskow RN. Preservation of implant esthetics: soft tissue and restorative considerations. J Esthet Dent. 1996;8(1):12-19.
30. Sailer I, Zembic A, Jung RE, et al. Single-tooth implant reconstructions: esthetic factors influencing the decision between titanium and zirconia abutments in anterior regions. Eur J Esthet Dent. 2007;2(3):296-310.
31. Jung RE, Holderegger C, Sailer I, et al. The effect of all-ceramic and porcelain-fused-to-metal restorations on marginal peri-implant soft tissue color: a randomized controlled clinical trial. Int J Periodontics Restorative Dent. 2008;28(4):357-365.
32. Bressan E, Paniz G, Lops D, et al. Influence of abutment material on the gingival color of implant-supported all-ceramic restorations: a prospective multicenter study. Clin Oral Implants Res. 2011;22(6):631-637.

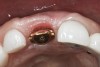
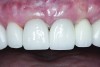 crown was cemented on the definitive abutment.">
crown was cemented on the definitive abutment.">