You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
Historically, evaluations of implant success focused exclusively on the ability of the implant to osseointegrate and maintain a functional prosthesis.1 However, as implant technology and understanding of the biology underlying osseointegration have evolved, the goal of contemporary implant dentistry has shifted. Today the faithful re-creation of what nature originally provided, both in terms of function and esthetics, has become the paramount objective.
Complicating that objective is the fact that resorption of alveolar bone is a common sequela of tooth loss, most notably within the first year.2-4 Along with developmental anomalies, acute or chronic pathology, and trauma, longstanding ridge resorption can limit the clinician’s ability to place implants in a way that will optimize the restorative results. Alternatively, implants may be placed in anatomically less favorable positions. However, a review of the literature reveals a consensus that improper implant positioning can lead to off-axis loads that in turn have been associated with biomechanical problems, such as screw loosening or fractures of the screw, implant, and/or implant collar.5,6 Other potential clinical prosthetic consequences include a nonideal emergence profile, prosthetic and porcelain fractures, poor screw-hole positioning, and occlusal discrepancies, such as cross-bite.
Proper bone-augmentation strategies incorporated within a treatment plan can enable the clinician to avoid such difficulties. Common regenerative indications include extraction sockets, horizontally and vertically atrophic ridges, implant-associated defects, and other anatomical structures that may inhibit proper implant positioning. Grafting techniques that have been well-documented to be successful in the treatment of such defects include ridge splitting and expansion;7,8 guided bone regeneration (GBR);9,10 distraction osteogenesis;11 onlay, inlay, and veneer grafts;12-14 nerve lateralization;15 and sinus augmentation.16
While successes obtained by using the four traditional bone-graft materials (autograft, allograft, xenograft, and alloplast) have all been well-documented, autogenous grafts historically have been considered the gold standard. Such bone, harvested either from extraoral or intraoral sites, has been thought to possess a higher osteoinductive potential and thus a greater ability to increase new bone formation. Use of this material also avoids any question of antigenicity or introduction of a foreign pathogen.
This article challenges the notion that autogenous block grafts should be the gold standard for traditional dental implant-associated bone regeneration. In both the literature and the experiences of the author, autogenous block grafts are associated with higher complication rates, greater resorption, and lower implant success rates than some allograft alternatives.
Autogenous Bone
More Complications
Autogenous block grafts often have sequelae that are specific to the donor site. Grafts harvested from the iliac crest, a form of endochondral bone, have been associated with significant morbidity.17 Such grafts also require patient hospitalization for graft procurement, which in turn increases costs. While iliac crest bone may play a role in the reconstruction of larger craniofacial cases, its value in the realm of implant reconstruction has become negligible.18Using autogenous block grafts of intramembranous histogenesis from intraoral sources, such as the ramus and mandibular symphysis, avoids the need for patient hospitalization. Moreover, such bone has been shown to have a lower rate of resorption19 and better revascularization20 than endochondral bone. However, routine postoperative complications associated with the harvesting of intramembranous block grafts include morbidity, parasthesia, anesthesia, and neurosensory changes to the proximal teeth and tissue.13,14,18,21,22 Other potential risks include edema, ptosis, incision dehiscence, infection, and even mortality.
Greater Resorption
Beyond these concerns, the total volume of bone gained from block grafting has fallen into question. A review of the literature reveals resorption rates ranging from 0% to 25% at the time of implant placement, with a further loss of up to 60% at the time of abutment connection.23-26 Using biologic barriers and adding xenograft material at autogenous bone-block grafting sites have been reported to reduce the total resorption rate.27,28 However, the average total gain in deficient vertical ridges was only about 5 mm in one 2005 human study.26 A comprehensive review by Gielkens et al29 of 182 articles found insufficient evidence to conclude that the use of a barrier membrane is effective in preventing onlay bone-graft resorption.Implant Success
Clinicians sometimes confound ridge-augmentation success with implant success. Yet the two are not synonymous, and implant success remains the ultimate goal. A systematic review of applicable data from 1980 to 2005 by Aghaloo and Moy30 reported findings of statistically significant reduced implant survival rates in sites grafted with autogenous bone block, compared with other regenerative techniques. They documented an implant survival rate of iliac crest grafts of 74.7%, as compared with a 95.5% survival rate for GBR.Allografts
An alternative to autogenous block grafts is allograft material applied in combination with a barrier membrane.31,32 The use of this commercially available material requires less time, ensures that an unlimited quantity of graft material will be available, and eliminates the need for a second surgical site with its attendant discomfort for the patient. Although some dental practitioners have considered all allograft material to be homogenous, numerous reports in the literature have demonstrated the unreliability of this assumption. The donor, tissue bank, and means by which the allograft is processed all can affect the allograft’s osseoinductive potential and the response of the recipient site to its introduction.33-35
Variation also exists in the way allograft materials are delivered. Allografts often take the form of particulate material; however, to increase the handling characteristics, some have been combined with carriers. Two established orthopedic graft materials have recently been adapted for oral and maxillofacial grafting applications: Each combines demineralized allograft bone with a carrier (lecithin in one case and collagen in the other) that facilitates handling.
In addition to improving the ease of handling, combining the demineralized bone particles with the carriers may also increase osseoinductivity at the graft site. When Han et al36 compared the osseoinductivity of delipidated demineralized bone to that of demineralized bone mixed with lecithin, they confirmed that removing lipids from demineralized bone can significantly inhibit osseoinduction, yet adding purified phosphatidylcholine (lecithin) appeared to restore the osseoinductive activity and enhance biologic activity above that of a standard demineralized bone preparation. The inductivity of both the lecithin-based putty and the collagen-based paste has been verified through established inductivity tests.
Following the principles of GBR and using a barrier membrane can enhance the likelihood of obtaining optimal results with the inductive allograft paste and/or putty.31 The ideal properties of a barrier for GBR procedures include the ability to exclude unwanted epithelial cells and maintain a space for appropriate cells (periodontal ligament cells, bone cells, and/or cementoblasts) to repopulate the wounded area. Although nonresorbable membranes have yielded successful results,37 their drawbacks include the fact that they require a second surgical entry, entailing increased patient cost, discomfort, and psychologic stress. Increased tissue trauma and wound-healing complications, such as membrane exposures, infection, bacterial contamination, and poor regenerative outcomes, have been associated with nonresorbable membranes.38-40
In contrast, resorbable membranes avoid the drawbacks associated with the need for a second membrane-removal surgery. The benefits of using collagen as the membrane material include the fact that collagen:
- is biocompatible;41,42
- provokes no significant immunogenic reactions;42
- has been shown to have a chemotactic effect on periodontal ligament cells43,44 and gingival fibroblasts; and
- promotes osteoblast proliferation, as well as increased secretion of transforming growth factor-β1, a growth factor involved in bone remodeling.45
Noncross-linked resorbable collagen membranes often foster a very rapid vascularization, with excellent tissue reaction subsequent to the vascularization.46,47 However, the resorption profile of noncross-linked collagen is roughly 4 to 8 weeks, and in the absence of complications, a statistically significant increase in bone formation can occur in the 8 to 16 weeks after grafting.48 To prolong the absorption profile of collagen membranes, various cross-linking techniques have been developed. Such membranes, while semipermeable and allowing for an exchange of nutrients, have been demonstrated to exclude epithelium and connective tissue for 6 months49 and, in the author’s experience, may do so for 1 year or more. Cross-linked collagen membranes thus have the potential to provide most of the benefits of nonresorbable membranes, without their drawbacks.
The following clinical cases illustrate the use of a cross-linked resorbable collagen membrane in conjunction with an allograft putty or paste.
Case Examples
Case 1
A 45-year-old man presented in the wake of a fall from a four-story scaffolding structure. His right lateral and left central incisors had been evulsed at the time of the accident, while his right central and left lateral incisors had intruded apically beyond the alveolar housing and fractured at the cementoenamel junction.
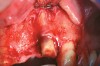
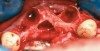
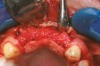
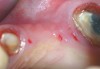
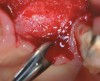
A full mucoperiosteal flap was reflected (Figure 1), and the residual roots of the two damaged teeth were extracted atraumatically, preserving the residual alveolar housing (Figure 2). Although thin, the facial plate was maintained to act as a supportive structure for the grafting material and membrane.
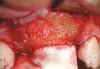
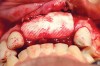
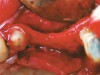
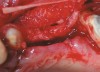
RegenerOss™ Allograft Putty (BIOMET 3i™, Palm Beach Gardens, FL) was molded to form the contours of the desired future ridge (Figure 3), and a 20-mm x 30-mm Ossix® Plus membrane (Colbar LifeScience, Ltd, Herzlya, Israel) was trimmed and adapted to cover the graft material (Figure 4). Periosteal relaxing incisions were made, and passive primary closure was obtained.
The patient received 1 g of amoxicillin before treatment and was instructed to continue taking 500 mg three times daily for 7 days. He was sent home with a removable partial that had been extensively relieved. Follow-up at 1 and 3 weeks and then monthly found unremarkable healing, with primary closure maintained throughout the healing period.
At 6 months postsurgery, re-entry revealed a notable increase in the dimensions of the ridge, with approximately 8 mm to 10 mm of bone gained horizontally and 3 mm to 4 mm vertically (Figure 5). Two implants were placed. Histologic examination of a 2-mm x 4-mm trephine bone core harvested just adjacent to the implant site revealed well-vascularized bone marrow and newly formed bone (Figure 6).
Case 2
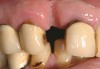
A 57-year-old woman presented seeking an implant-supported restoration to replace a failing bridge from her right maxillary second molar to her first premolar (Figure 7). Those teeth had been extracted traumatically more than 30 years ago.
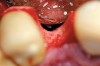
A full-thickness incision was made. Flap reflection revealed a narrow residual ridge with significant apical undercuts (Figure 8). Regenaform® paste (Exactech, Inc, Gainesville, FL) was molded on the buccal aspect of the ridge, as well as crestally. A 20-mm x 30-mm Ossix Plus membrane was trimmed and adapted to cover the graft material (Figure 9). Periosteal releasing incisions were made, and passive primary closure was obtained.
After 7 months of unremarkable healing, surgical re-entry revealed more than 10 mm of newly formed horizontal bone (Figure 10).
Case 3
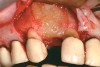
A 45-year-old male who smoked presented in the wake of a blunt trauma to the face. The right lateral incisor had been evulsed, with obvious damage to the facial plate (Figure 11). All other anterior teeth were salvageable.
Reflection of a full-thickness flap revealed complete loss of the facial plate (Figure 12). Approximately 1 cm3 of RegenerOss Allograft Putty was expressed into the defect and molded (Figure 13), then covered with an Ossix Plus membrane (Figure 14). Extensive periosteal-releasing incisions were used in conjunction with mesial- and distal-releasing incisions to obtain passive primary closure.
Although the patient was noncompliant with a smoking cessation program, the soft-tissue healing at 1 and 6 months was unremarkable. Re-entry at 6 months revealed evidence of the residual biologic membrane, along with excellent osseous in-fill.
At the time of implant placement, a 2-mm trephine drill was used to remove an 8-mm bone core, which was placed in formalin. After enlargement of the osteotomy and placement of an 11.5-mm Certain® PREVAIL® Implant (BIOMET 3i) (Figure 15), the core was sent out for histologic examination. The histology revealed that the graft material was well-encapsulated, with excellent vascularity and newly formed bone (Figure 16).
Discussion
During osteotomy preparation, 3 to 6 months after augmenting oral sites with the verified inductive bone-graft materials, the author has typically noted excellent volumetric fill. Histologic examination of these sites has also confirmed excellent bone formation and vascularity. However, the density of the bone is often type 2 to 3 (according to the Lekholm and Zarb scale). An explanation for the seeming disparity between the positive histologic results and the apparent soft nature of the new bone may lie in the nature of demineralized allograft material. When Cammack et al50 compared mineralized and demineralized freeze-dried bone allograft, they found no statistical difference in percentages of new bone formed at sites grafted with each material. However, significantly less residual demineralized bone was found in localized ridge-augmentation sites, suggesting that the mineralized material might take longer to resorb and might account for the apparent harder feel of sites grafted with it. A biopsy of a demineralized bone graft site also showed many more marrow spaces when compared with a mineralized bone graft site. Yet the differences between the two appeared to have no bearing on the success or failure of the graft of implant.
The possibilities for combining the allograft paste and putty with mineralized bone or other augmentation materials raises the possibility of achieving new bone that is harder while at the same time taking advantage of the increased osseoinductivity and superior ease of handling afforded by the allograft paste and putty.
Conclusion
Proper bone-augmentation strategies are essential for re-creating natural function and esthetics, the paramount objective in implant dentistry today. Although autogenous grafts historically have been considered the gold standard among grafting materials, they are associated with higher complication rates, greater resorption, and lower implant success than some allograft alternatives. New commercially available allograft materials used in conjunction with established regenerative protocols result in comparable success rates with minimum sequelae often associated with block grafts.
Part II of this article will examine the characteristics and applications of established orthopedic allograft bone in oral rehabilitation.
Disclosure
The author is a consultant for BIOMET 3i.
References
1. Albrektsson T, Zarb G, Worthington P, et al. The long-term efficacy of currently used dental implants: a review and proposed criteria of success. Int J Oral Maxillofac Implants. 1986;1(1):11-25.
2. Schropp L, Wenzel A, Kostopoulos L, et al. Bone healing and soft tissue contour changes following single-tooth extraction: a clinical and radiographic 12-month prospective study. Int J Periodontics Restorative Dent. 2003;23(4):313-327.
3. Simon B, Von Hagen S, Deasy MJ, et al. Changes in alveolar bone height and width following ridge augmentation using bone graft and membranes. J Periodontol. 2000;71(11):1774-1791.
4. Nevins M, Camelo M, De Paoli S, et al. A study of the fate of buccal wall of extraction sockets of teeth with prominent roots. Int J Periodontics Restorative Dent. 2006;26(1):19-29.
5. Rangert B, Jemt T, Jörneus L. Forces and moments on Branemark implants. Int J Oral Maxillofac Implants. 1989;4(3):241-247.
6. Khraisat A, Abu-Hammad O, Dar-Odeh N, et al. Abutment screw loosening and bending resistance of external hexagon implant system after lateral cyclic loading. Clin Implant Dent Relat Res. 2004;6(3):157-164.
7. Duncan JM, Westwood RM. Ridge widening for the thin maxilla: a clinical report. Int J Oral Maxillofac Implants. 1997;12(2):224-227.
8. Scipioni A, Bruschi G, Calesini G. The edentulous ridge expansion technique: a five-year study. Int J Periodontics Restorative Dent. 1994;14(5):451-459.
9. Mellonig JT, Nevins M. Guided bone regeneration of bone defects associated with implants: an evidence-based outcome assessment. Int J Periodontics Restorative Dent. 1995;15(2):168-185.
10. Zitzmann N, Naef R, Schärer P. Resorbable versus nonresorbable membranes in combination with Bio-Oss for guided bone regeneration [published erratum appears in: Int J Oral Maxillofac Implants. 1998;13(4):576]. Int J Oral Maxillofac Implants. 1997;12(6):844-852.
11. Urbani G, Lombardo G, Santi E, et al. Distraction osteogenesis to achieve mandibular vertical bone regeneration: a case report. Int J Periodontics Restorative Dent. 1999;19(4):321-331.
12. Proussaefs P, Lozada J. The use of intraorally harvested autogenous block grafts for vertical alveolar ridge augmentation: a human study. Int J Periodontics Restorative Dent. 2005;25(4):351-363.
13. Misch CM. Comparison of intraoral donor sites for onlay grafting prior to implant placement. Int J Oral Maxillofac Implants. 1997;12(6):767-776.
14. Pikos MA. Block autografts for localized ridge augmentation: part I. The posterior maxilla. Implant Dent. 1999;8(3):279-285.
15. Jensen J, Reiche-Fischel O, Sindet-Pederson S. Nerve transposition and implant placement in the atrophic posterior mandibular alveolar ridge. J Oral Maxillofac Surg. 1994;52(7):662-668.
16. Boyne PJ, James RA. Grafting of the maxillary sinus floor with autogenous marrow and bone. J Oral Surg. 1980;38(8):613-616.
17. Rudman RA. Prospective evaluation of morbidity associated with iliac crest harvest for alveolar cleft grafting. J Oral Maxillofac Surg. 1997;55(3):2219-2223.
18. Schwartz-Arad D, Levin L, Sigal L. Surgical success of intraoral autogenous block onlay grafting for alveolar ridge augmentation. Implant Dent. 2005;14(2):131-138.
19. Smith JD, Abramsson M. Membranous vs endochondrial bone autografts. Arch Otolaryngol. 1974;99(3):203-205.
20. Burchardt H. The biology of bone graft repair. Clin Orthop Relat Res. 1983;(174):28-42.
21. Raghoebar GM, Louwerverse C, Kalk WW, et al. Morbidity of chin bone harvesting. Clin Oral Implants Res. 2001;12(5):503-507.
22. Clavero J, Lundgren S. Ramus or chin grafts for maxillary sinus inlay and local onlay augmentation: comparison of donor site morbidity and complications. Clin Implant Dent Relat Res. 2003;5(3):154-160.
23. Misch CM, Misch CE, Resnik RR, et al. Reconstruction of maxillary alveolar defects with mandibular symphysis grafts for dental implants: a preliminary procedural report. Int J Oral Maxillofac Implants. 1992;7(3):360-366.
24. Widmark G, Andersson B, Ivanoff CJ. Mandibular bone graft in the anterior maxilla for single-tooth implants. Presentation of surgical method. Int J Oral Maxillofac Surg. 1997;26(2):106-109.
25. McAllister BS, Haghighat K. Bone augmentation techniques. J Periodontol. 2007:78(3):377-396.
26. Proussaefs P, Lozada J. The use of intraorally harvested autogenous block grafts for vertical alveolar ridge augmentation: a human study. Int J Periodontics Restorative Dent. 2005;25(4):351-363.
27. Proussaefs P, Lozada J, Rohrer MD. A clinical and histologic evaluation of a block onlay graft in conjunction with autogenous particulate and inorganic bovine mineral (Bio-Oss): a case report. Int J Periodontics Restorative Dent. 2002;22(6):567-573.
28. Jardini MA, De Marco AC, Lima LA. Early healing pattern of autogenous bone grafts with and without e-PTFE membranes: a histomorphometric study in rats. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100(6):666-673.
29. Gielkens PF, Bos RF, Raghoebar GM, et al. Is there evidence that barrier membranes prevent bone resorption in autologous bone grafts during the healing period? A systematic review. Int J Oral Maxillofac Implants. 2007;22(3):390-398.
30. Aghaloo TL, Moy PK. Which hard tissue augmentation techniques are the most successful in furnishing bony support for implant placement [published erratum appears in: Int J Oral Maxillofac Implants. 2008;23(1):56]. Int J Oral Maxillofac Implants. 2007;22(Suppl):49-70.
31. Mellonig JT, Nevins M. Guided bone regeneration of bone defects associated with implants: an evidence-based outcome assessment. Int J Periodontics Restorative Dent. 1995;15(2):168-185.
32. Simion M, Jovanovic SA, Trisi P, et al. Vertical ridge augmentation around dental implants using a membrane technique and autogenous bone or allografts in humans. Int J Periodontics Restorative Dent. 1998;18(1):8-23.
33. Becker W, Becker BE, Caffesse R. A comparison of demineralized freeze-dried bone and autologous bone to induce bone formation in human extraction sockets [published erratum appears in: J Periodontol. 1995;66(4):309]. J Periodontol. 1994:65(12):1128-1133.
34. Becker W, Clokie C, Sennerby L, et al. Histologic findings after implantation and evaluation of different graft materials and titanium micro screws into extraction sockets: case report. J Periodontol. 1998:69(4):414-421.
35. Schwartz Z, Sommers A, Mellonig JT, et al. Ability of commercial demineralized freeze-dried bone allograft to induce new bone formation is dependent on donor age but not gender. J Periodontol. 1996;69(4):470-478.
36. Han B, Tang B, Nimni ME. Combined effects of phosphatidylcholine and demineralized bone matrix on bone induction. Connect Tissue Res. 2003;44(3-4):160-166.
37. Jovanovic SA, Nevins M. Bone formation utilizing titanium-reinforced barrier membranes. Int J Periodontics Restorative Dent. 1995;15(1):56-69.
38. von Arx T, Hardt N, Wallkamm B. The TIME technique: a new method for localized alveolar ridge augmentation prior to placement of dental implants. Int J Oral Maxillofac Implants. 1996;11(3):387-394.
39. Machtei EE. The effect of membrane exposure on the outcome of regenerative procedures in humans: a meta-analysis. J Periodontol. 2001;72(4):512-516.
40. Simion M, Baldoni M, Rossi P, et al. A comparative study of the effectiveness of e-PTFE membranes with and without early exposure during the healing period. Int J Periodontics Restorative Dent. 1994;14(2):166-180.
41. Quteish D, Singrao S, Dolby AE. Light and electron microscopic evaluation of biocompatibility, resorption, and penetration characteristics of human collagen graft material. J Clin Periodontol. 1991;18(5):305-311.
42. Sableman EE. Biology, biotechnology and biocompatability of collagen. In: Williams DF, ed. Biocompatability of Tissue Analogs. 1st ed. Boca Raton, FL: CRC Press, Inc; 1985:27.
43. Postlethwaite AE, Seyer JM, Kang AH. Chemotactic attraction of human fibroblasts to type I, II, III collagens and collagen derived peptides. Proc Natl Acad Sci U S A. 1978;75(2):871-875.
44. Yaffe A, Ehrich J, Shoshan S. Restoration of periodontal attachment employing collagen solution in the dog. J Periodontal. 1984;55(11):623-628.
45. Marinucci L, Lilli C, Baroni T, et al. In vitro comparison of bioabsorbable and non-resorbable membranes in bone regeneration. J Periodontol. 2001;72(6):753-759.
46. Rothamel D, Schwartz F, Sagar M, et al. Biodegradation of different cross-linked collagen membranes: an experimental study in the rat. Clin Oral Implants Res. 2005;16(3):369-378.
47. Bunyaratavej P, Wang HL. Collagen membranes: a review. J Periodontol. 2001;72(2):215-229.
48. Bornstein MM, Bosshardt D, Bruser D. Effect of two different bioabsorbable collagen membranes on guided bone regeneration: a comparative histomorphometric study in the dog mandible. J Periodontol. 2007;78(10):1943-1953.
49. Freidman A, Strietzel FP, Maretzki B, et al. Observations on a new collagen barrier membrane in 16 consecutively treated patients. Clinical and histologic findings. J Periodontol. 2001;72:1616-1623.
50. Cammack GV Jr, Nevins M, Clem DS III, et al. Histologic evaluation of mineralized and demineralized freeze-dried bone allograft for ridge and sinus augmentations. Int J Periodontics Restorative Dent. 2005;25(3):231-237.
About the Author
John Lupovici, DDS, Private Practice, New York, New York